System Chemistry in Catalysis: Facing the Next Challenges in Production of Energy Vectors and Environmental Remediation
Abstract
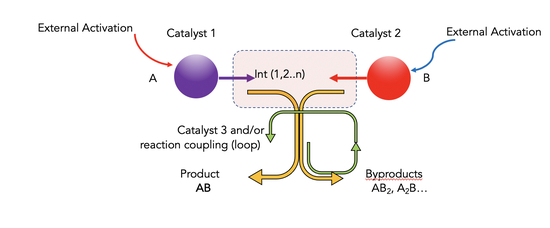
:1. Introduction
2. Systemic Catalysts: Design Strategies and Selected Examples
2.1. Dynamics of Factors that Influence the Performances of a Catalyst
2.2. Selected Examples of Systemic Catalytic Strategies
2.3. Manipulating the Local Microenvironment: The Case of Copper Catalysts for CO2 Reduction and Related Reactions
2.4. Turning Problems into Opportunities: The Case of NiFe for Methane Dry Reforming and Chemical Looping
2.5. Systemic Approaches for Oxygen Evolution Reaction (OER)
2.6. Environmental Remediation
3. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Ludlow, R.F.; Otto, S. Systems chemistry. Chem. Soc. Rev. 2008, 37, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ashkenasy, G.; Hermans, T.M.; Otto, S.; Taylor, A.F. Systems chemistry. Chem. Soc. Rev. 2017, 46, 2543–2554. [Google Scholar] [CrossRef]
- Semenov, S.N.; Kraft, L.J.; Ainla, A.; Zhao, M.; Baghbanzadeh, M.; Campbell, V.E.; Kang, K.; Fox, J.M.; Whitesides, G.M. Autocatalytic, bistable, oscillatory networks of biologically relevant organic reactions. Nature 2016, 537, 656–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissette, A.J.; Fletcher, S.P. Mechanisms of autocatalysis. Angew. Chem. Int. Ed. 2013, 52, 12800–12826. [Google Scholar] [CrossRef] [PubMed]
- Blackmond, D.G. An Examination of the Role of Autocatalytic Cycles in the Chemistry of Proposed Primordial Reactions. Angew. Chem. 2009, 121, 392–396. [Google Scholar] [CrossRef]
- Yoshida, R.; Murase, Y. Self-oscillating surface of gel for autonomous mass transport. Colloids Surf. B Biointerfaces 2012, 99, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Tabata, O.; Hirasawa, H.; Aoki, S.; Yoshida, R.; Kokufuta, E. Ciliary motion actuator using self-oscillating gel. Sens. Actuators A Phys. 2002, 95, 234–238. [Google Scholar] [CrossRef]
- Maeda, S.; Hara, Y.; Sakai, T.; Yoshida, R.; Hashimoto, S. Self-walking gel. Adv. Mater. 2007, 19, 3480–3484. [Google Scholar] [CrossRef]
- Chen, I.C.; Kuksenok, O.; Yashin, V.V.; Balazs, A.C.; Van Vliet, K.J. Mechanical resuscitation of chemical oscillations in Belousov-Zhabotinsky gels. Adv. Funct. Mater. 2012, 22, 2535–2541. [Google Scholar] [CrossRef]
- Vassalini, I.; Alessandri, I. Spatial and Temporal Control of Information Storage in Cellulose by Chemically Activated Oscillations. ACS Appl. Mater. Interfaces 2015, 7, 28708–28713. [Google Scholar] [CrossRef]
- Yang, T.H.; Zhou, S.; Gilroy, K.D.; Figueroa-Cosme, L.; Lee, Y.H.; Wu, J.M.; Xia, Y. Autocatalytic surface reduction and its role in controlling seed-mediated growth of colloidal metal nanocrystals. Proc. Natl. Acad. Sci. USA 2017, 114, 13619–13624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maity, I.; Dev, D.; Basu, K.; Wagner, N.; Ashkenasy, G. Signaling in Systems Chemistry: Programing Gold Nanoparticles Formation and Assembly Using a Dynamic Bistable Network. Angew. Chem. Int. Ed. 2020, 59, 1–7. [Google Scholar] [CrossRef]
- Twilton, J.; Le, C.C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1. [Google Scholar] [CrossRef]
- Allen, A.E.; MacMillan, D.W.C. Synergistic catalysis: A powerful synthetic strategy for new reaction development. Chem. Sci. 2012, 3, 633–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Neri, S.; Prins, L.J. Enhanced catalytic activity under non-equilibrium conditions. Nat. Nanotechnol. 2020, 15, 868–874. [Google Scholar] [CrossRef]
- Lubrano Lavadera, M.; Song, Y.; Sabia, P.; Herbinet, O.; Pelucchi, M.; Stagni, A.; Faravelli, T.; Battin-Leclerc, F.; De Joannon, M. Oscillatory Behavior in Methane Combustion: Influence of the Operating Parameters. Energy Fuels 2018, 32, 10088–10099. [Google Scholar] [CrossRef]
- Tiemersma, T.P.; Kolkman, T.; Kuipers, J.A.M.; van Sint Annaland, M. A novel autothermal reactor concept for thermal coupling of the exothermic oxidative coupling and endothermic steam reforming of methane. Chem. Eng. J. 2012, 203, 223–230. [Google Scholar] [CrossRef]
- Rofer-DePoorter, C.K. A Comprehensive Mechanism for the Fischer-Tropsch Synthesis. Chem. Rev. 1981, 81, 447–474. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Wells, P.; Gianolio, D. Synchrotron radiation techniques in catalytic science. Phys. Chem. Chem. Phys. 2020, 22, 18745–18746. [Google Scholar] [CrossRef]
- Alessandri, I. Enhancing raman scattering without plasmons: Unprecedented sensitivity achieved by TiO2 shell-based resonators. J. Am. Chem. Soc. 2013, 135, 5541–5544. [Google Scholar] [CrossRef]
- Alessandri, I.; Vassalini, I.; Bertuzzi, M.; Bontempi, N.; Memo, M.; Gianoncelli, A. “RaMassays”: Synergistic Enhancement of Plasmon-Free Raman Scattering and Mass Spectrometry for Multimodal Analysis of Small Molecules. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessandri, I.; Carletti, L.; Ferroni, M.; De Angelis, C.; Vassalini, I. Bioinspired self-similar all-dielectric antennas: Probing the effect of secondary scattering centres by Raman spectroscopy. Mater. Adv. 2020, 1, 2443–2449. [Google Scholar] [CrossRef]
- Bontempi, N.; Vassalini, I.; Alessandri, I. All-dielectric core/shell resonators: From plasmon-free SERS to multimodal analysis. J. Raman Spectrosc. 2018, 49, 943–953. [Google Scholar] [CrossRef]
- Bontempi, N.; Vassalini, I.; Danesi, S.; Alessandri, I. ZORRO: Zirconium oxide resonators for all-in-one Raman and whispering-gallery-mode optical sensing. Chem. Commun. 2017, 53, 10382–10385. [Google Scholar] [CrossRef] [PubMed]
- Vassalini, I.; Rotunno, E.; Lazzarini, L.; Alessandri, I. “stainless” Gold Nanorods: Preserving Shape, Optical Properties, and SERS Activity in Oxidative Environment. ACS Appl. Mater. Interfaces 2015, 7, 18794–18802. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, N.; Vassalini, I.; Danesi, S.; Ferroni, M.; Donarelli, M.; Colombi, P.; Alessandri, I. Non-Plasmonic SERS with Silicon: Is It Really Safe? New Insights into the Optothermal Properties of Core/Shell Microbeads. J. Phys. Chem. Lett. 2018, 9, 2127–2132. [Google Scholar] [CrossRef]
- DeRita, L.; Resasco, J.; Dai, S.; Boubnov, A.; Thang, H.V.; Hoffman, A.S.; Ro, I.; Graham, G.W.; Bare, S.R.; Pacchioni, G.; et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 2019, 18, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Bañares, M.A.; Depero, L.E.; Ferroni, M.; Fornasiero, P.; Gennari, F.C.; Hickey, N.; Martinez-Huerta, M.V.; Montini, T. Structural investigation of Ce2Zr2O8 after redox treatments which lead to low temperature reduction. Top. Catal. 2006, 41, 35–42. [Google Scholar] [CrossRef]
- Vassalini, I.; Alessandri, I. Switchable stimuli-responsive heterogeneous catalysis. Catalysts 2018, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Moussallem, I.; Jörissen, J.; Kunz, U.; Pinnow, S.; Turek, T. Chlor-alkali electrolysis with oxygen depolarized cathodes: History, present status and future prospects. J. Appl. Electrochem. 2008, 38, 1177–1194. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, A.; Li, F.; Hung, S.F.; Nam, D.H.; Gabardo, C.M.; Wang, Z.; Xu, Y.; Ozden, A.; Rasouli, A.S.; et al. Efficient Methane Electrosynthesis Enabled by Tuning Local CO2 Availability. J. Am. Chem. Soc. 2020, 142, 3525–3531. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Thevenon, A.; Rosas-Hernández, A.; Wang, Z.; Li, Y.; Gabardo, C.M.; Ozden, A.; Dinh, C.T.; Li, J.; Wang, Y.; et al. Molecular tuning of CO2-to-ethylene conversion. Nature 2020, 577, 509–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mara, P.B.; Wilde, P.; Benedetti, T.M.; Andronescu, C.; Cheong, S.; Gooding, J.J.; Tilley, R.D.; Schuhmann, W. Cascade Reactions in Nanozymes: Spatially Separated Active Sites inside Ag-Core-Porous-Cu-Shell Nanoparticles for Multistep Carbon Dioxide Reduction to Higher Organic Molecules. J. Am. Chem. Soc. 2019, 141, 14093–14097. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.P.; Zhang, X.L.; Gao, F.Y.; Zheng, Y.R.; Niu, Z.Z.; Yu, X.; Liu, R.; Wu, Z.Z.; Qin, S.; Chi, L.P.; et al. Protecting Copper Oxidation State via Intermediate Confinement for Selective CO2 Electroreduction to C2+ Fuels. J. Am. Chem. Soc. 2020, 142, 6400–6408. [Google Scholar] [CrossRef]
- Jeon, H.S.; Timosnenko, J.; Scholten, F.; Sinev, I.; Herzog, A.; Haase, F.T.; Cuenya, B.R. Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction. J. Am. Chem. Soc. 2020, 141, 19879–19887. [Google Scholar] [CrossRef] [Green Version]
- Albinsson, D.; Boje, A.; Nilsson, S.; Tiburski, C.; Hellman, A.; Ström, H.; Langhammer, C. Copper catalysis at operando conditions—bridging the gap between single nanoparticle probing and catalyst-bed-averaging. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, Y.; Ma, J.; Wang, C.; Sheng, S.; Cheng, C.; Li, M.; Han, L.; Zhou, L.; Cai, Z.; et al. An Artificial Electrode/Electrolyte Interface for CO2 Electroreduction by Cation Surfactant Self-Assembly. Angew. Chem. Int. Ed. 2020, 132, 19257–19263. [Google Scholar] [CrossRef]
- Kim, D.; Kley, C.S.; Li, Y.; Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl. Acad. Sci. USA 2017, 114, 10560–10565. [Google Scholar] [CrossRef] [Green Version]
- Marimuthu, A.; Zhang, J.; Linic, S. Tuning Selectivity in Propylene Epoxidation by Plasmon Mediated Photo-Switching of Cu Oxidation State. Science 2013, 339, 1590–1593. [Google Scholar] [CrossRef] [Green Version]
- Vassalini, I.; Alessandri, I. “The phactalysts”: Carbon nanotube/TiO2 composites as phototropic actuators for wireless remote triggering of chemical reactions and catalysis. Nanoscale 2017, 9, 11446–11451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; Van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Chung, D.Y.; Kim, H.; Chung, Y.H.; Lee, M.J.; Yoo, S.J.; Bokare, A.D.; Choi, W.; Sung, Y.E. Inhibition of CO poisoning on Pt catalyst coupled with the reduction of toxic hexavalent chromium in a dual-functional fuel cell. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, D.; Donat, F.; Abdala, P.M.; Kim, S.M.; Kierzkowska, A.M.; Müller, C.R. Reversible Exsolution of Dopant Improves the Performance of Ca2Fe2O5 for Chemical Looping Hydrogen Production. ACS Appl. Mater. Interfaces 2019, 11, 18276–18284. [Google Scholar] [CrossRef]
- Hosseini, D.; Donat, F.; Kim, S.M.; Bernard, L.; Kierzkowska, A.M.; Müller, C.R. Redox-Driven Restructuring of FeMnZr-Oxygen Carriers Enhances the Purity and Yield of H2 in a Chemical Looping Process. ACS Appl. Energy Mater. 2018, 1, 1294–1303. [Google Scholar] [CrossRef]
- Donat, F.; Müller, C.R. CO2-free conversion of CH4 to syngas using chemical looping. Appl. Catal. B Environ. 2020, 278. [Google Scholar] [CrossRef]
- Marxer, D.; Furler, P.; Takacs, M.; Steinfeld, A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ. Sci. 2017, 10, 1142–1149. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.; Steinfeld, A. Concentrating solar thermal power and thermochemical fuels. Energy Environ. Sci. 2012, 5, 9234–9245. [Google Scholar] [CrossRef]
- Garcia, A.C.; Touzalin, T.; Nieuwland, C.; Perini, N.; Koper, M.T.M. Enhancement of Oxygen Evolution Activity of Nickel Oxyhydroxide by Electrolyte Alkali Cations. Angew. Chem. Int. Ed. 2019, 58, 12999–13003. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Gorlin, Y.; Chung, C.J.; Benck, J.D.; Nordlund, D.; Seitz, L.; Weng, T.C.; Sokaras, D.; Clemens, B.M.; Jaramillo, T.F. Understanding interactions between manganese oxide and gold that lead to enhanced activity for electrocatalytic water oxidation. J. Am. Chem. Soc. 2014, 136, 4920–4926. [Google Scholar] [CrossRef]
- Gorlin, M.; De Araujo, J.F.; Schmies, H.; Bernsmeier, D.; Dresp, S.; Gliech, M.; Jusys, Z.; Chernev, P.; Kraehnert, R.; Dau, H.; et al. Tracking catalyst redox states and reaction dynamics in ni-fe oxyhydroxide oxygen evolution reaction electrocatalysts: The role of catalyst support and electrolyte pH. J. Am. Chem. Soc. 2017, 139, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Frydendal, R.; Busch, M.; Halck, N.B.; Paoli, E.A.; Krtil, P.; Chorkendorff, I.; Rossmeisl, J. Enhancing activity for the oxygen evolution reaction: The beneficial interaction of gold with manganese and cobalt oxides. ChemCatChem 2015, 7, 149–154. [Google Scholar] [CrossRef]
- Vassalini, I.; Borgese, L.; Mariz, M.; Polizzi, S.; Aquilanti, G.; Ghigna, P.; Sartorel, A.; Amendola, V.; Alessandri, I. Enhanced Electrocatalytic Oxygen Evolution in Au–Fe Nanoalloys. Angew. Chem. Int. Ed. 2017, 56, 6589–6593. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Burke, M.S.; Kast, M.G.; Fan, J.; Danilovic, N.; Boettcher, S.W. Fe (Oxy)hydroxide Oxygen Evolution Reaction Electrocatalysis: Intrinsic Activity and the Roles of Electrical Conductivity, Substrate, and Dissolution. Chem. Mater. 2015, 27, 8011–8020. [Google Scholar] [CrossRef]
- Yeo, B.S.; Bell, A.T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2011, 133, 5587–5593. [Google Scholar] [CrossRef]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T.M. MnOx/IrOx as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef] [Green Version]
- Dresp, S.; Ngo Thanh, T.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energy Environ. Sci. 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Salmistraro, M.; Schwartzberg, A.; Bao, W.; Depero, L.E.; Weber-Bargioni, A.; Cabrini, S.; Alessandri, I. Triggering and monitoring plasmon-enhanced reactions by optical nanoantennas coupled to photocatalytic beads. Small 2013, 9, 3301–3307. [Google Scholar] [CrossRef]
- Salmistraro, M.; Sassolini, S.; Weber-Bargioni, A.; Cabrini, S.; Alessandri, I. Fabrication of gold nanoantennas on SiO2/TiO2 core/shell beads to study photon-driven surface reactions. Microelectron. Eng. 2015, 143, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Ravetz, B.D.; Pun, A.B.; Churchill, E.M.; Congreve, D.N.; Rovis, T.; Campos, L.M. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 2019, 565, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Proppe, A.H.; Li, Y.C.; Aspuru-Guzik, A.; Berlinguette, C.P.; Chang, C.J.; Cogdell, R.; Doyle, A.G.; Flick, J.; Gabor, N.M.; van Grondelle, R.; et al. Bioinspiration in light harvesting and catalysis. Nat. Rev. Mater. 2020, 5, 828–846. [Google Scholar] [CrossRef]
- Ng, B.J.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S.P. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Vassalini, I.; Gjipalaj, J.; Crespi, S.; Gianoncelli, A.; Mella, M.; Ferroni, M.; Alessandri, I. Alginate-Derived Active Blend Enhances Adsorption and Photocatalytic Removal of Organic Pollutants in Water. Adv. Sustain. Syst. 2020, 4, 1–11. [Google Scholar] [CrossRef]
- Vassalini, I.; Ribaudo, G.; Gianoncelli, A.; Casula, M.F.; Alessandri, I. Plasmonic hydrogels for capture, detection and removal of organic pollutants. Environ. Sci. Nano 2020. [Google Scholar] [CrossRef]
- Wei, H.; Loeb, S.K.; Halas, N.J.; Kim, J.-H.; David Waite, T.; Wang, J. Plasmon-enabled degradation of organic micropollutants in water by visible-light illumination of Janus gold nanorods. Proc. Natl. Acad. Sci. USA 2020, 27, 15473–15481. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Li, X.; Guan, D.; Li, Y.; Zheng, X.; Zhao, M.; Shan, C.; Pan, B. Autocatalytic Decomplexation of Cu(II)-EDTA and Simultaneous Removal of Aqueous Cu(II) by UV/Chlorine. Environ. Sci. Technol. 2019, 53, 2036–2044. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fern, C. Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Schlexer Lamoureux, P.; Winther, K.T.; Garrido Torres, J.A.; Streibel, V.; Zhao, M.; Bajdich, M.; Abild-Pedersen, F.; Bligaard, T. Machine Learning for Computational Heterogeneous Catalysis. ChemCatChem 2019, 11, 3581–3601. [Google Scholar] [CrossRef] [Green Version]
- Enman, L.J.; Stevens, M.B.; Dahan, M.H.; Nellist, M.R.; Toroker, M.C.; Boettcher, S.W. Operando X-ray Absorption Spectroscopy Shows Iron Oxidation is Concurrent with Oxygen Evolution in Cobalt–Iron (Oxy)hydroxide Electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 12840–12844. [Google Scholar] [CrossRef]
- Dou, J.; Sun, Z.; Opalade, A.A.; Wang, N.; Fu, W.; Tao, F. Operando chemistry of catalyst surfaces during catalysis. Chem. Soc. Rev. 2017, 46, 2001–2027. [Google Scholar] [CrossRef] [PubMed]
- Centi, G.; Cejka, J. Needs and Gaps for Catalysis in Addressing Transitions in Chemistry and Energy from a Sustainability Perspective. ChemSusChem 2019, 12, 621–632. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessandri, I.; Vassalini, I. System Chemistry in Catalysis: Facing the Next Challenges in Production of Energy Vectors and Environmental Remediation. Catalysts 2021, 11, 64. https://doi.org/10.3390/catal11010064
Alessandri I, Vassalini I. System Chemistry in Catalysis: Facing the Next Challenges in Production of Energy Vectors and Environmental Remediation. Catalysts. 2021; 11(1):64. https://doi.org/10.3390/catal11010064
Chicago/Turabian StyleAlessandri, Ivano, and Irene Vassalini. 2021. "System Chemistry in Catalysis: Facing the Next Challenges in Production of Energy Vectors and Environmental Remediation" Catalysts 11, no. 1: 64. https://doi.org/10.3390/catal11010064
APA StyleAlessandri, I., & Vassalini, I. (2021). System Chemistry in Catalysis: Facing the Next Challenges in Production of Energy Vectors and Environmental Remediation. Catalysts, 11(1), 64. https://doi.org/10.3390/catal11010064