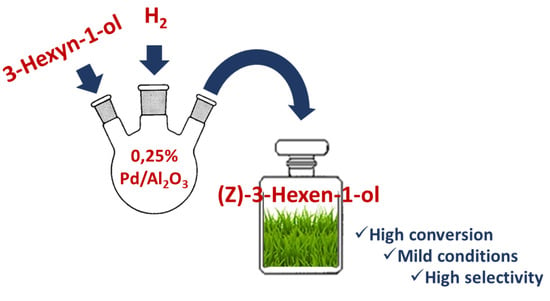
A Smart Heterogeneous Catalyst for Efficient, Chemo- and Stereoselective Hydrogenation of 3-Hexyn-1-ol
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Preparation of Cat 1 and Cat 1*
3.3. Preparation of Cat 2
3.4. Hydrogenation of 3-hexyn-1-ol (I)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blaser, H.U.; Malan, C.; Pugin, B.; Spindler, F.; Steiner, H.; Studer, M. Selective Hydrogenation for Fine Chemicals: Recent Trends and New Developments. Adv. Synth. Catal. 2003, 345, 103–151. [Google Scholar] [CrossRef]
- Vilé, G.; Albani, D.; Almora-Barrios, N.; López, N.; Pérez-Ramírez, J. Advances in the Design of Nanostructured Catalysts for Selective Hydrogenation. ChemCatChem 2016, 8, 21–33. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective Hydrogenation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef] [PubMed]
- Campanati, M.; Fornasari, G.; Vaccari, A. Fundamentals in the preparation of heterogeneous catalysts. Catal. Today 2003, 77, 299–314. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Lindlar, H. Ein neuer Katalysator für selektive Hydrierungen. Helv. Chim. Acta 1952, 35, 446–450. [Google Scholar] [CrossRef]
- Molnár, Á.; Sárkány, A.; Varga, M. Hydrogenation of carbon–carbon multiple bonds: Chemo-, regio- and stereo-selectivity. J. Mol. Catal. 2001, 173, 185–221. [Google Scholar] [CrossRef]
- Anderson, J.A.; Mellor, J.; Wells, R.P.K. Pd catalysed hexyne hydrogenation modified by Bi and by Pb. J. Catal. 2009, 261, 208–216. [Google Scholar] [CrossRef]
- Maccarrone, M.J.; Lederhosa, C.R.; Torresa, G.; Betti, C.; Coloma-Pascual, F.; Quiroga, M.E.; Yori, J.C. Partial hydrogenation of 3-hexyne over low-loaded palladium mono and bimetallic catalysts. Appl. Catal. A Gen. 2012, 441–442, 90–98. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Cárdenas-Lizana, F.; Dessimoz, A.-L.; Kiwi-Minsker, L. Modern Trends in Catalyst and Process Design for Alkyne Hydrogenations. ACS Catal. 2012, 2, 1773–1786. [Google Scholar] [CrossRef]
- Witte, P.T.; Boland, S.; Kirby, F.; van Maanen, R.; Bleeker, B.F.; de Winter, D.A.M.; Post, J.A.; Geus, J.W.; Berben, P.H. NanoSelect Pd Catalysts: What Causes the High Selectivity of These Supported Colloidal Catalysts in Alkyne Semi—Hydrogenation? ChemCatChem 2013, 5, 582–587. [Google Scholar] [CrossRef]
- Mitsudome, T.; Takahashi, Y.; Ichikawa, S.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Metal–Ligand Core–Shell Nanocomposite Catalysts for the Selective Semihydrogenation of Alkynes. Angew. Chem. Int. Ed. 2013, 52, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Yarulin, A.; Yuranov, I.; Cárdenas-Lizana, F.; Alexander, D.T.L.; Kiwi-Minsker, L. How to increase the selectivity of Pd-based catalyst in alkynol hydrogenation: Effect of second metal. Appl. Catal. A Gen. 2014, 478, 186–193. [Google Scholar] [CrossRef]
- Vilé, G.; Almira-Barrios, N.; Mitchell, S.; López, N.; Pérez-Ramírez, J. From the Lindlar Catalyst to Supported Ligand-Modified Palladium Nanoparticles: Selectivity Patterns and Accessibility Constraints in the Continuous-Flow Three-Phase Hydrogenation of Acetylenic Compounds. Chem. Eur. J. 2014, 20, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Liu, H.J.; Lo, H.K.; Copéret, C. Silica-Supported Cu Nanoparticle Catalysts for Alkyne Semihydrogenation: Effect of Ligands on Rates and Selectivity. J. Am. Chem. Soc. 2016, 138, 16502–16507. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, X.; Takale, B.S.; Yamamoto, Y.; Zhang, W.; Bao, M. Highly Selective Semihydrogenation of Alkynes to Alkenes by Using an Unsupported Nanoporous Palladium Catalyst: No Leaching of Palladium into the Reaction Mixture. ACS Catal. 2017, 7, 8296–8303. [Google Scholar] [CrossRef]
- Delgado, J.A.; Benkirane, O.; Claver, C.; Curulla-Ferréc, D.; Godard, C. Advances in the preparation of highly selective nanocatalysts for the semi-hydrogenation of alkynes using colloidal approaches. Dalton Trans. 2017, 46, 12381–12403. [Google Scholar] [CrossRef] [Green Version]
- Ibhadon, A.O.; Kansal, S.K. The Reduction of Alkynes Over Pd-Based Catalyst Materials—A Pathway to Chemical Synthesis. J. Chem. Eng. Process Technol. 2018, 9. [Google Scholar] [CrossRef]
- Maazaoui, R.; Abderrahim, R.; ChemLa, F.; Ferreira, F.; Perez-Luna, A.; Jackowski, O. Catalytic Chemoselective and Stereoselective Semihydrogenation of Alkynes to E-Alkenes Using the Combination of Pd Catalyst and ZnI2. Org. Lett. 2018, 20, 7544–7549. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Rassolov, A.V.; Mashkovsky, I.S.; Stakheev, A.Y. Highly Selective Pd–Cu/α-Al2O3 Catalysts for Liquid-Phase Hydrogenation: The Influence of the Pd: Cu Ratio on the Structure and Catalytic Characteristics. Kinet. Catal. 2018, 59, 601–609. [Google Scholar] [CrossRef]
- Da Silva, F.P.; Fiorio, J.L.; Gonçalves, R.V.; Teixeira-Neto, E.; Rossi, L.M. Synergic Effect of Copper and Palladium for Selective Hydrogenation of Alkynes. Ind. Eng. Chem. 2018, 57, 16209–16216. [Google Scholar] [CrossRef]
- Swamy, K.K.C.; Reddy, A.S.; Sandeep, K.; Kalyani, A. Advances in chemoselective and/or stereoselective semihydrogenation of alkynes. Tetrahedron Lett. 2018, 59, 419–429. [Google Scholar] [CrossRef]
- Buxaderas, E.; Volpe, M.A.; Radivoy, G. Selective Semi-Hydrogenation of Terminal Alkynes Promoted by Bimetallic Cu-Pd Nanoparticles. Synthesis 2019, 51, 1466–1472. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Yao, Z.; Zhou, Q.; Zhuang, G.; Zhong, X.; Deng, S.; Li, X.; Wang, J. Optimizing Alkyne Hydrogenation Performance of Pd on Carbon in Situ Decorated with Oxygen-Deficient TiO2 by Integrating the Reaction and Diffusion. ACS Catal. 2019, 9, 10656–10667. [Google Scholar] [CrossRef]
- Guan, Q.; Zhang, J.; He, L.; Miao, R.; Shi, Y.; Ning, P. Selective Hydrogenation of Acetylene to Ethylene over the Surface of Sub-2 nm Pd Nanoparticles in Miscanthus sinensis-Derived Microporous Carbon Tubes. ACS Sustain. Chem. Eng. 2020, 8, 11638–11648. [Google Scholar] [CrossRef]
- Spee, M.P.R.; Boersma, J.; Meijer, M.D.; Slagt, M.Q.; van Koten, G.; Geus, J.W. Selective Liquid-Phase Semihydrogenation of Functionalized Acetylenes and Propargylic Alcohols with Silica-Supported Bimetallic Palladium−Copper Catalysts. J. Org. Chem. 2001, 66, 1647–1656. [Google Scholar] [CrossRef] [Green Version]
- Stoll, M.; Rouvé, A. Synthèse du cis-β, γ-hexénol (hexénol naturel). Helv. Chim. Acta 1938, 21, 1542–1547. [Google Scholar] [CrossRef]
- Witte, P.T.; de Groen, M.; de Rooij, R.M.; Bakermans, P.; Donkervoort, H.G.; Berben, P.H.; Geus, J.W. Highly active and selective precious metal catalysts by use of the reduction-deposition method. Stud. Surf. Sci. Catal. 2010, 175, 135–143. [Google Scholar] [CrossRef]
- Liguori, F.; Barbaro, P. Green semi-hydrogenation of alkynes by Pd@borate monolith catalysts under continuous flow. J. Catal. 2014, 311, 212–220. [Google Scholar] [CrossRef]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the Key Aroma Compounds in Pink Guava (Psidium guajava L.) by Means of Aroma Re-engineering Experiments and Omission Tests. J. Agric. Food Chem. 2009, 57, 2882–2888. [Google Scholar] [CrossRef]
- (Z)-hex-3-en-1-ol. Available online: http://www.thegoodscentscompany.com/data/rw1005932.html (accessed on 22 December 2020).
- Tassini, R.; La Sorella, G.; Montin, D.; Paganelli, S.; Baldi, F.; Piccolo, O. Sintesi sostenibili con CO. La Chimica e l’Industria 2012, 94, 157. [Google Scholar]
- Paganelli, S.; Tassini, R.; Rathod, V.D.; Onida, B.; Fiorilli, S.; Piccolo, O. A Low Rhodium Content Smart Catalyst for Hydrogenation and Hydroformylation Reactions. Catal. Lett. 2020. [Google Scholar] [CrossRef]
- Piccolo, O.; Verrazzani, A. US 7087548, (Chemi SpA). 2006. Available online: https://worldwide.espacenet.com/publicationDetails/originalDocument?FT=D&date=20060808&DB=EPODOC&locale=en_EP&CC=US&NR=7087548B2&KC=B2&ND=4 (accessed on 22 December 2020).









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganelli, S.; Angi, A.; Pajer, N.; Piccolo, O. A Smart Heterogeneous Catalyst for Efficient, Chemo- and Stereoselective Hydrogenation of 3-Hexyn-1-ol. Catalysts 2021, 11, 14. https://doi.org/10.3390/catal11010014
Paganelli S, Angi A, Pajer N, Piccolo O. A Smart Heterogeneous Catalyst for Efficient, Chemo- and Stereoselective Hydrogenation of 3-Hexyn-1-ol. Catalysts. 2021; 11(1):14. https://doi.org/10.3390/catal11010014
Chicago/Turabian StylePaganelli, Stefano, Alessio Angi, Nicolò Pajer, and Oreste Piccolo. 2021. "A Smart Heterogeneous Catalyst for Efficient, Chemo- and Stereoselective Hydrogenation of 3-Hexyn-1-ol" Catalysts 11, no. 1: 14. https://doi.org/10.3390/catal11010014
APA StylePaganelli, S., Angi, A., Pajer, N., & Piccolo, O. (2021). A Smart Heterogeneous Catalyst for Efficient, Chemo- and Stereoselective Hydrogenation of 3-Hexyn-1-ol. Catalysts, 11(1), 14. https://doi.org/10.3390/catal11010014