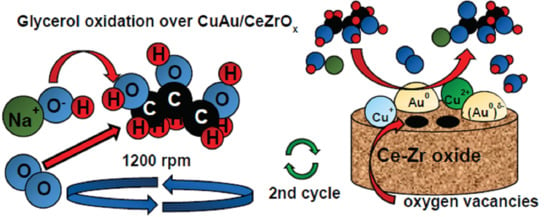
The Application of Copper-Gold Catalysts in the Selective Oxidation of Glycerol at Acid and Basic Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Characterisation
2.2. The Influence of Selected Conditions on the Catalytic Activity and Selectivity
2.2.1. The Influence of Oxygen Source and pH Solution
2.2.2. The Influence of Catalyst Amount
2.2.3. The Effect of Oxygen Pressure
2.2.4. The Influence of Base Content
2.2.5. The Catalytic Stability
2.3. The Changes in Materials after Glycerol Oxidation
2.3.1. UV-Vis Study
2.3.2. XRD Measurements
2.3.3. TEM and STEM-EDXS Analysis
2.3.4. XPS Measurements
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Preparation of Pure Zirconia, ZrO2 and Mixed Cerium-Zirconium Oxide, CeZrOx
3.1.2. Preparation of Pure Ceria, CeO2
3.1.3. Preparation of Catalysts Modified with Gold Species
3.1.4. Modification of Gold Catalysts with Copper Species
3.2. Catalysts Characterisation
3.2.1. X-ray Diffraction (XRD)
3.2.2. The Adsorption and Desorption of Nitrogen
3.2.3. Ultra-Violet (UV-Vis) Spectroscopy
3.2.4. X-ray Photoelectron Spectroscopy (XPS)
3.2.5. Transmission Electron Microscopy (TEM)
3.2.6. Scanning Transmission Electron Microscopy Combined with Energy Dispersive X-ray Spectroscopy (STEM-EDXS)
3.3. Catalytic Test—Reaction of Glycerol Oxidation
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C.D. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Ten Dam, J.; Hanefeld, U. Renewable chemicals: Dehydroxylation of glycerol and polyols. ChemSusChem 2001, 4, 1017–1034. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Chen, X.; Shen, Y. Commodity chemicals derived from glycerol, an important biorefinery feedstock. Chem. Rev. 2008, 108, 5253–5277. [Google Scholar] [CrossRef]
- Kimura, H. Selective oxidation of glycerol on a platinum-bismuth catalyst by using a fixed bed reactor. Appl. Catal. A Gen. 1993, 105, 147–158. [Google Scholar] [CrossRef]
- Dodekatos, G.; Tüysüz, H. Effect of Post-Treatment on Structure and Catalytic Activity of CuCo-based Materials for Glycerol Oxidation. ChemCatChem 2017, 9, 610–619. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Hutchings, G.J. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide. Chem. Commun. 2002, 696–697. [Google Scholar] [CrossRef]
- Demirel-Gülen, S.; Lucas, M.; Waerna, J.; Salmi, T.; Murzin, D.; Claus, P. Reaction kinetics and modelling of the gold catalysed glycerol oxidation. Top. Catal. 2007, 44, 299–305. [Google Scholar] [CrossRef]
- Ketchie, W.C.; Murayama, M.; Davis, R.J. Promotional effect of hydroxyl on the aqueous phase oxidation of carbon monoxide and glycerol over supported Au catalysts. Top. Catal. 2007, 44, 307–317. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketchie, W.C.; Fang, L.Y.; Wong, M.S.; Murayama, M.; Davis, R.J. Influence of gold particle size on the aqueous-phase oxidation of carbon monoxide and glycerol. J. Catal. 2007, 250, 94–101. [Google Scholar] [CrossRef]
- Villa, A.; Veith, G.M.; Ferri, D.; Weidenkaff, A.; Perry, K.A.; Campisi, S.; Prati, L. NiO as a peculiar support for metal nanoparticles in polyols oxidation. Catal. Sci. Technol. 2013, 3, 394–399. [Google Scholar] [CrossRef]
- Komanoya, T.; Suzuki, A.; Nakajima, K.; Kitano, M.; Kamata, K.; Hara, M. A Combined Catalyst of Pt Nanoparticles and TiO2 with Water-Tolerant Lewis Acid Sites for One-Pot Conversion of Glycerol to Lactic Acid. ChemCatChem 2016, 8, 1094–1099. [Google Scholar] [CrossRef]
- Redina, E.A.; Kirichenko, O.A.; Greish, A.A.; Kucherov, A.V.; Tkachenko, O.P.; Kapustin, G.I.; Mishin, I.V.; Kustov, L.M. Preparation of bimetallic gold catalysts by redox reaction on oxide-supported metals for green chemistry applications. Catal. Today 2015, 246, 216–231. [Google Scholar] [CrossRef]
- Purushothaman, R.K.P.; van Haveren, J.; van Es, D.S.; Melián-Cabrera, I.; Meeldijk, J.D.; Heeres, H.J. An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support. Appl. Catal. B Environ. 2014, 147, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Zhang, S.; Li, H.; Ren, Y.; Liu, H. Efficient Synthesis of Lactic Acid by Aerobic Oxidation of Glycerol on Au–Pt/TiO2 Catalysts. Chem. Eur. J. 2010, 16, 7368–7371. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Lennon, D.; Porta, F.; Prati, L.; Villa, A. Effect of Particle Size on Monometallic and Bimetallic (Au,Pd)/C on the Liquid Phase Oxidation of Glycerol. Catal. Lett. 2006, 108, 147–153. [Google Scholar] [CrossRef]
- Villa, A.; Campisi, S.; Mohammed, K.M.H.; Dimitratos, N.; Vindigni, F.; Manzoli, M.; Jones, W.; Bowker, M.; Hutchings, G.J.; Prati, L. Tailoring the selectivity of glycerol oxidation by tuning the acid–base properties of Au catalysts. Catal. Sci. Technol. 2015, 5, 1126–1132. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, C.; Brett, G.; Divitini, G.; Ducati, C.; Hutchings, G.J.; Mantle, M.D.; Gladden, L.F. Increased Affinity of Small Gold Particles for Glycerol Oxidation over Au/TiO2 Probed by NMR Relaxation Methods. ACS Catal. 2017, 7, 4235–4241. [Google Scholar] [CrossRef]
- Díaz, J.A.; Skrzyńska, E.; Zaid, S.; Girardon, J.; Capron, M.; Dumeignil, F.; Fongarland, P. Kinetic modelling of the glycerol oxidation in the liquid phase: Comparison of Pt, Au and Ag AS active phases. J. Chem. Technol. Biotechnol. 2017, 92, 2267–2275. [Google Scholar] [CrossRef]
- Yang, G.-Y.; Ke, Y.-H.; Ren, H.-F.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. The conversion of glycerol to lactic acid catalyzed by ZrO2-supported CuO catalysts. Chem. Eng. J. 2016, 283, 759–767. [Google Scholar] [CrossRef]
- Abdullah, R.; Abdullah, A.Z. Effect of catalyst to glycerol ratio in the production of lactic acid via hydrothermal reaction using calcium oxide and strontium oxide catalysts. AIP Conf. Proc. 2018, 2030, 020197. [Google Scholar] [CrossRef]
- Palacio, R.; Torres, S.; Lopez, D.; Hernandez, D. Selective glycerol conversion to lactic acid on Co3O4/CeO2 catalysts. Catal. Today 2018, 302, 196–202. [Google Scholar] [CrossRef]
- De Clercq, R.; Dusselier, M.; Makshin, E.; Sels, B.F. Catalytic Gas-Phase Production of Lactide from Renewable Alkyl Lactates. Angew. Chem. Int. Ed. 2018, 57, 3074–3078. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Kiely, C.J.; Hutchings, G.J. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Phys. Chem. Chem. Phys. 2003, 5, 1329–1336. [Google Scholar] [CrossRef]
- Porta, F.; Prati, L. Selective oxidation of glycerol to sodium glycerate with gold-on-carbon catalyst: An insight into reaction selectivity. J. Catal. 2004, 224, 397–403. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Williams, C.T.; Monnier, J.R. Selective liquid-phase oxidation of glycerol over Au–Pd/C bimetallic catalysts prepared by electroless deposition. Appl. Catal. A Gen. 2014, 475, 161–168. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Wang, X.; Mou, C.Y.; Zhang, T. Au-Cu Alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem. Commun. 2008, 27, 3187–3189. [Google Scholar] [CrossRef] [PubMed]
- Zavrazhnov, S.A.; Esipovich, A.L.; Zlobin, S.Y.; Belousov, A.S.; Vorotyntsev, A.V. Mechanism Analysis and Kinetic Modelling of Cu NPs Catalysed Glycerol Conversion into Lactic Acid. Catalysts 2019, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Arcanjo, M.R.A.; da Silva, I.J.; Cavalcante, C.L.; Iglesias, J.; Morales, G.; Paniagua, M.; Melero, J.A.; Vieira, R.S. Glycerol valorization: Conversion to lactic acid by heterogeneous catalysis and separation by ion exchange chromatography. Biofuels Bioprod. Biorefin. 2019, 14, 357–370. [Google Scholar] [CrossRef]
- Eriksson, C.J.P.; Saarenmaa, T.P.S.; Bykov, I.L.; Heino, P.U. Acceleration of ethanol and acetaldehyde oxidation by D-glycerate in rats. Metabolism 2007, 56, 895–898. [Google Scholar] [CrossRef]
- Rosseto, R.; Tcacenco, C.M.; Ranganathan, R.; Hajdu, J. Synthesis of phosphatidylcholine analogues derived from glyceric acid: A new class of biologically active phospholipid compounds. Tetrahedron Lett. 2008, 49, 3500–3503. [Google Scholar] [CrossRef] [Green Version]
- Prati, L.; Rossi, M. Gold on Carbon as a New Catalyst for Selective Liquid Phase Oxidation of Diols. J. Catal. 1998, 176, 552–560. [Google Scholar] [CrossRef]
- Prati, L.; Martra, G. New gold catalysts for liquid phase oxidation. Gold Bull. 1999, 32, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Wang, D.; Veith, G.M.; Prati, L. Bismuth as a modifier of Au–Pd catalyst: Enhancing selectivity in alcohol oxidation by suppressing parallel reaction. J. Catal. 2012, 292, 73–80. [Google Scholar] [CrossRef]
- Kondrat, S.A.; Miedziak, P.J.; Douthwaite, M.; Brett, G.L.; Davies, T.E.; Morgan, D.J.; Edwards, J.K.; Knight, D.W.; Kiely, C.J.; Taylor, S.H.; et al. Base-Free Oxidation of Glycerol Using Titania-Supported Trimetallic Au–Pd–Pt Nanoparticles. ChemSusChem 2014, 7, 1326–1334. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Zhao, Y.; Yu, B.; Chen, S.; Li, Y.; Hao, L.; Liu, Z. Selective oxidation of glycerol to lactic acid under acidic conditions using AuPd/TiO2 catalyst. Green Chem. 2013, 15, 1520–1525. [Google Scholar] [CrossRef]
- Zaid, S.; Skrzyńska, E.; Addad, A.; Nandi, S.; Jalowiecki-Duhamel, L.; Girardon, J.-S.; Capron, M.; Dumeignil, F. Development of silver based catalysts promoted by noble metal M (M = Au, Pd or Pt) for glycerol oxidation in liquid phase. Top. Catal. 2017, 60, 1072–1081. [Google Scholar] [CrossRef]
- Kaskow, I.; Decyk, P.; Sobczak, I. The effect of copper and silver on the properties of Au-ZnO catalyst and its activity in glycerol oxidation. Appl. Surf. Sci. 2018, 444, 197–207. [Google Scholar] [CrossRef]
- Frassoldati, A.; Pinel, C.; Besson, M. Promoting effect of water for aliphatic primary and secondary alcohol oxidation over platinum catalysts in dioxane/aqueous solution media. Catal. Today 2013, 173, 81–88. [Google Scholar] [CrossRef]
- Douthwaite, M.; Powell, N.; Taylor, A.; Ford, G.; López, J.M.; Solsona, B.; Yang, N.; Sanahuja-Parejo, O.; He, Q.; Morgan, D.J.; et al. Glycerol Selective Oxidation to Lactic Acid over AuPt Nanoparticles; Enhancing Reaction Selectivity and Understanding by Support Modification. ChemCatChem 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on platinum metal catalysts in aqueous solutions. Catal. Today 1994, 19, 247–283. [Google Scholar] [CrossRef]
- Gangwal, V.R.; van del Schaaf, J.; Kuster, B.F.M.; Schouten, J.C. Influence of pH on noble metal catalysed alcohol oxidation: Reaction kinetics and modelling. J. Catal. 2005, 229, 389–403. [Google Scholar] [CrossRef]
- Abad, A.; Almela, C.; Corma, C.; Garcia, H. Unique gold chemoselectivity for the aerobic oxidation of allylic alcohols. Tetrahedron 2006, 62, 6666–6672. [Google Scholar] [CrossRef]
- Hou, W.; Dehm, N.A.; Scott, R.W.J. Alcohol oxidations in aqueous solutions using Au, Pd, and bimetallic AuPd nanoparticle catalysts. J. Catal. 2008, 253, 22–27. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Kiely, C.J. Strategies for the synthesis of supported gold palladium nanoparticles with controlled morphology and composition. Acc. Chem. Res. 2013, 46, 1759–1772. [Google Scholar] [CrossRef]
- Marx, S.; Baiker, A. Beneficial Interaction of Gold and Palladium in Bimetallic Catalysts for the Selective Oxidation of Benzyl Alcohol. J. Phys. Chem. C 2009, 113, 6191–6201. [Google Scholar] [CrossRef]
- Kaizuka, K.; Miyamura, H.; Kobayashi, S. Remarkable effect of bimetallic nanocluster catalysts for aerobic oxidation of alcohols: Combining metals changes the activities and the reaction pathways to aldehydes/carboxylic acids or esters. J. Am. Chem. Soc. 2010, 132, 15096–15098. [Google Scholar] [CrossRef] [PubMed]
- Dimitratos, N.; Villa, A.; Wang, D.; Porta, F.; Su, D.; Prati, L. Pd and Pt catalysts modified by alloying with Au in the selective oxidation of alcohols. J. Catal. 2006, 244, 113–121. [Google Scholar] [CrossRef]
- Prati, L.; Villa, A.; Porta, F.; Wang, D.; Su, D. Single-phase gold/palladium catalyst: The nature of synergetic effect. Catal. Today 2007, 122, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.C.; Veith, G.M.; Allard, L.F.; Oyola, Y.; Overbury, S.H.; Dai, S. Silica-Supported Au–CuOx Hybrid Nanocrystals as Active and Selective Catalysts for the Formation of Acetaldehyde from the Oxidation of Ethanol. ACS Catal. 2012, 2, 2537–2546. [Google Scholar] [CrossRef]
- Zhao, G.F.; Hu, H.Y.; Deng, M.M.; Ling, M.; Lu, Y. Foam/fiber-structured catalysts: Non-dip-coating fabrication strategy and applications in heterogeneous catalysis. Green Chem. 2013, 13, 55–58. [Google Scholar] [CrossRef]
- Kaminski, P.; Ziolek, M.; van Bokhoven, J.A. Mesoporous cerium–zirconium oxides modified with gold and copper–synthesis, characterization and performance in selective oxidation of glycerol. RSC Adv. 2017, 7, 7801–7819. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, P.; Ziolek, M.; Campo, B.; Daturi, M. FTIR spectroscopic study of CO oxidation on bimetallic catalysts. Catal. Today 2015, 243, 218–227. [Google Scholar] [CrossRef]
- Kaminski, P.; Ziolek, M. Mobility of gold, copper and cerium species in Au, Cu/Ce, Zr-oxides and its impact on total oxidation of methanol. Appl. Catal. B Environ. 2016, 187, 328–341. [Google Scholar] [CrossRef]
- Dimitratos, N.; Villa, A.; Bianchi, C.L.; Prati, L.; Makkee, M. Gold on titania: Effect of preparation method in the liquid phase oxidation. Appl. Catal. A Gen. 2006, 311, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Prati, L.; Spontoni, P.; Gaiassi, A. From renewable to fine chemicals through selective oxidation: The case of glycerol. Top. Catal. 2009, 52, 288–296. [Google Scholar] [CrossRef]
- Veith, G.M.; Lupini, A.R.; Pennycook, S.J.; Villa, A.; Prati, L.; Dudney, N.J. Magnetron sputtering of gold nanoparticles onto WO3 and activated carbon. Catal. Today 2007, 122, 248–253. [Google Scholar] [CrossRef]
- Villa, A.; Gaiassi, A.; Rossetti, I.; Bianchi, C.L.; Benthem, K.; van Veith, G.M.; Prati, L. Au on MgAl2O4 spinels: The effect of support surface properties in glycerol oxidation. J. Catal. 2010, 275, 108–116. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Ronsein, G.E.; Corrêa, T.C.; Martinez, R.G.; Medeiros, M.H.G.; Di Mascio, P. Direct evidence of singlet molecular oxygen generation from peroxynitrate, a decomposition product of peroxynitrite. Dalton Trans. 2009, 5720–5729. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.X.; Zhao, Y.; Ma, S.C.; Liu, S.T. Decomposition Characteristics of Hydrogen Peroxide in Sodium Hydroxide Solution. Adv. Mater. Res. 2013, 610–613, 359–362. [Google Scholar] [CrossRef]
- Sobczak, I.; Kusior, A.; Grams, J.; Ziolek, M. The role of chlorine in the generation of catalytic active species located in Au-containing MCM-41 materials. J. Catal. 2007, 245, 259–266. [Google Scholar] [CrossRef]
- Sobczak, I.; Jagodzinska, K.; Ziolek, M. Glycerol oxidation on gold catalysts supported on group five metal oxides–A comparative study with other metal oxides and carbon based catalysts. Catal. Today 2010, 158, 121–129. [Google Scholar] [CrossRef]
- Escamilla-Perea, L.; Nava, R.; Pawelec, B.; Rosmaninho, M.G.; Peza-Ledesma, C.L.; Fierro, J.L.G. SBA-15-supported gold nanoparticles decorated by CeO2: Structural characteristics and CO oxidation activity. Appl. Catal. A Gen. 2010, 381, 42–53. [Google Scholar] [CrossRef]
- Laha, S.C.; Mukerjee, P.; Sainkar, S.R.; Kumar, R. Cerium containing MCM-41-type mesoporous materials and their acidic and redox catalytic properties. J. Catal. 2002, 207, 213–223. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Kharlanov, A.N.; Kochubey, D.I.; Bogdanchikova, N.; Stakheev, A.Y. Influence of modifying additives on electronic state of supported gold. J. Mol. Struct. 2002, 642, 129–136. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Kharlanov, A.N.; Bogdanchikova, N.E.; Tuzovskaya, I.V. Electronic state of gold in supported clusters. Eur. Phys. J. D 2003, 24, 307–309. [Google Scholar] [CrossRef]
- Pestryakov, A.; Tuzovskaya, I.; Smolentseva, E.; Bogdanchikova, N.; Jentoft, F.; Knop-Gericke, A. Formation of gold nanoparticles in zeolites. Int. J. Mod. Phys. B 2005, 19, 2321–2326. [Google Scholar] [CrossRef]
- Simakov, A.; Bogdanchikova, N.; Tuzovskaya, I.; Smoletseva, E.; Pestryakov, A.; Farias, M.; Avalos, M. Catalysts based on gold nanosized species incorporated into zeolites. In Complex Mediums VI: Light and Complexity; McCall, M.W., Dewar, G., Noginov, M.A., Eds.; SPIE: San Diego, CA, USA, 2005; Volume 5924, p. 101. ISBN 9780819459299. [Google Scholar]
- Smolentseva, E.; Bogdanchikova, N.; Simakov, A.; Pestryakov, A.; Tuzovskaya, I.; Avalos, M.; Farías, M.H.; Díaz, J.A.; Gurin, V. Influence of copper modifying additive on state of gold in zeolites. Surf. Sci. 2006, 600, 4256–4259. [Google Scholar] [CrossRef]
- Smolentseva, E.; Bogdanchikova, N.; Simakov, A.; Pestryakov, A.; Avalos, M.; Farias, M.H.; Tompos, A.; Gurin, V. Catalytic activity of gold nanoparticles incorporated into modified zeolites. J. Nanosci. Nanotechnol. 2007, 7, 1882–1886. [Google Scholar] [CrossRef]
- Tuzovskaya, I.V.; Simakov, A.V.; Pestryakov, A.N.; Bogdanchikova, N.E.; Gurin, V.V.; Farías, M.H.; Tiznado, H.J.; Avalos, M. Co-existence of various active gold species in Au-mordenite catalyst for CO oxidation. Catal. Commun. 2007, 8, 977–980. [Google Scholar] [CrossRef]
- Costa, V.V.; Estrada, M.; Demidova, Y.; Prosvirin, I.; Kriventsov, V.; Cotta, R.F.; Fuentes, S.; Simakov, A.; Gusevskaya, E.V. Gold nanoparticles supported on magnesium oxide as catalysts for the aerobic oxidation of alcohols under alkali-free conditions. J. Catal. 2012, 292, 148–156. [Google Scholar] [CrossRef]
- Feldheim, D.L.; Foss, C.A. Metal Nanoparticles: Synthesis, Characterization and Applications; Dekker, M., Ed.; CRC Press: New York, NY, USA; Basel, Switzerland, 2002; ISBN 0-8247-0604-8. [Google Scholar]
- Feng, R.; Li, M.; Liu, J. Synthesis of core-shell Au@Pt nanoparticles supported on Vulcan XC-72 carbon and their electrocatalytic activities for methanol oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 406, 6–12. [Google Scholar] [CrossRef]
- Galindo-Hernández, F.; Wang, J.A.; Gómez, R.; Bokhimi, X.; Lartundo, L.; Mantilla, A. Structural modifications in Au/Al2O3–CeO2 mixed oxides as a function of Ce4+ content and its effects in the mineralization of the herbicide diuron. J. Photochem. Photobiol. A Chem. 2012, 243, 23–32. [Google Scholar] [CrossRef]
- Guzmán, C.; del Ángel, G.; Gómez, R.; Galindo-Hernández, F.; Ángeles-Chavez, C. Degradation of the herbicide 2,4-dichlorophenoxyacetic acid over Au/TiO2–CeO2 photocatalysts: Effect of the CeO2 content on the photoactivity. Catal. Today 2011, 166, 146–151. [Google Scholar] [CrossRef]
- Irie, H.; Miura, S.; Kamiya, K.; Hashimoto, K. Efficient visible light-sensitive photocatalysts: Grafting Cu(II) ions onto TiO2 and WO3 photocatalysts. Chem. Phys. Lett. 2008, 457, 202–205. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 Nanocomposites as Risk-Reduction Materials in Indoor Environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Chary, K.V.R.; Sagar, G.V.; Naresh, D.; Seela, K.K.; Sridhar, B. Characterization and reactivity of copper oxide catalysts supported on TiO2-ZrO2. J. Phys. Chem. B 2005, 109, 9437–9444. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liu, L.; Lv, Y.; Zhu, J.; Wan, H.; Liu, B.; Gao, F.; Wang, X.; Dong, L.; Chen, Y. Surface structure characteristics of CuO/Ti0.5Sn0.5O2 and its activity for CO oxidation. J. Mol. Catal. A Chem. 2012, 365, 87–94. [Google Scholar] [CrossRef]
- Liu, Z.; Amiridis, M.D.; Chen, Y. Characterization of CuO Supported on Tetragonal ZrO2 Catalysts for N2O Decomposition to N2. J. Phys. Chem. B 2005, 109, 1251–1255. [Google Scholar] [CrossRef]
- Sobczak, I.; Wolski, Ł. Au–Cu on Nb2O5 and Nb/MCF supports–Surface properties and catalytic activity in glycerol and methanol oxidation. Catal. Today 2015, 254, 72–82. [Google Scholar] [CrossRef]
- Kaminski, P.; Sobczak, I.; Decyk, P.; Ziolek, M.; Roth, W.J.; Campo, B.; Daturi, M. Zeolite MCM-22 Modified with Au and Cu for Catalytic Total Oxidation of Methanol and Carbon Monoxide. J. Phys. Chem. C 2013, 117, 2147–2159. [Google Scholar] [CrossRef]
- Kaminski, P.; Ziolek, M. Surface and catalytic properties of Ce-, Zr-, Au-, Cu-modified SBA-15. J. Catal. 2014, 312, 249–262. [Google Scholar] [CrossRef]









| Catalyst | Conv., % | Selectivity, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLA 2 | GCA 2 | FA 2 | TA 2 | CO2 | ||||||||
| 15 mg | 30 mg | 15 mg | 30 mg | 15 mg | 30 mg | 15 mg | 30 mg | 15 mg | 30 mg | 15 mg | 30 mg | |
| CuAu/CeO2 | 53 | 72 | 57 | 68 | 9 | 17 | 12 | 16 | 3 | - | 19 | - |
| CuAu/CeZrOx | 66 | 80 | 79 | 79 | 7 | 7 | 11 | 14 | 2 | - | 1 | - |
| CuAu/ZrO2 | 64 | 80 | 68 | 70 | 7 | 11 | 23 | 16 | 2 | - | - | 3 |
| Catalyst | Conv., % | Selectivity, % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLA 2 | GCA 2 | FA 2 | TA 2 | LA 2 | CO2 | |||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| Au/CeO2 | 31 | 62 | 18 | 59 | 1 | 3 | 1 | 6 | - | 3 | - | - | 81 | 30 |
| Au/CeZrOx | 32 | 25 | 18 | 16 | 1 | 1 | 1 | 1 | - | - | - | - | 81 | 82 |
| Au/ZrO2 | 19 | 35 | 4 | 3 | - | - | - | - | - | - | - | - | 96 | 97 |
| CuAu/CeO2 | 79 | 79 | 54 | 76 | 40 | 8 | - | 12 | - | 3 | - | - | 6 | - |
| CuAu/CeZrOx | 85 | 73 | 77 | 81 | 6 | - | 8 | 6 | - | 2 | - | 12 | 10 | - |
| CuAu/ZrO2 | 83 | 62 | 63 | 93 | 16 | - | 4 | 5 | - | 2 | - | - | 17 | - |
| Catalyst | Distribution of Species (Estimated Using a XPS Method), % | |||||||
|---|---|---|---|---|---|---|---|---|
| Au Species | Cu Species | Ce Species | ||||||
| (Au0)δ- | Au0 | Auδ+ | Cu0 | Cu+ | Cu2+ | Ce3+ | Ce4+ | |
| Au/CeO2 before | - | 100 | - | - | - | - | 29 | 71 |
| Au/CeO2 after | - | 100 | - | - | - | - | 34 | 66 |
| Au/CeZrOx before | - | 84 | 16 | - | - | - | 24 | 76 |
| Au/CeZrOx after | - | 92 | 8 | - | - | - | 41 | 59 |
| Au/ZrO2 before | - | 89 | 11 | - | - | - | - | - |
| Au/ZrO2 after | - | 93 | 7 | - | - | - | - | - |
| CuAu/CeO2 before | - | 96 | 4 | - | 83 | 17 | 23 | 77 |
| CuAu/CeO2 after | 18 | 82 | - | 10 | 52 | 38 | 24 | 76 |
| CuAu/CeZrOx before | 19 | 81 | - | - | 91 | 9 | 18 | 82 |
| CuAu/CeZrOx after | 8 | 91 | 1 | 16 | 64 | 20 | 39 | 61 |
| CuAu/ZrO2 before | 16 | 71 | 13 | - | 86 | 14 | - | - |
| CuAu/ZrO2 after | 12 | 88 | - | 21 | 33 | 46 | - | - |
| Catalyst | Effect of Selected Factors on the Glycerol Conversion in Its Oxidation, % | |||||||
|---|---|---|---|---|---|---|---|---|
| pH Solution and Oxygen Source | Oxygen Pressure, Bar | Molar Ratio (NaOH/Glycerol) | ||||||
| NaOH and O2 | NaOH and H2O2 | HNO3 and O2 | HNO3 and H2O2 | 3 | 6 | 1:1 | 2:1 | |
| CuAu/CeO2 | 72 | 31 | 18 | 28 | 53 | 72 | 76 | 72 |
| CuAu/CeZrOx | 80 | 47 | 20 | 20 | 60 | 80 | 63 | 80 |
| CuAu/ZrO2 | 80 | 40 | 17 | 16 | 49 | 80 | 79 | 80 |
| main product | GLA | CO2 | CO2 | CO2 | GLA/CO2 | GLA | GLA | GLA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminski, P. The Application of Copper-Gold Catalysts in the Selective Oxidation of Glycerol at Acid and Basic Conditions. Catalysts 2021, 11, 94. https://doi.org/10.3390/catal11010094
Kaminski P. The Application of Copper-Gold Catalysts in the Selective Oxidation of Glycerol at Acid and Basic Conditions. Catalysts. 2021; 11(1):94. https://doi.org/10.3390/catal11010094
Chicago/Turabian StyleKaminski, Piotr. 2021. "The Application of Copper-Gold Catalysts in the Selective Oxidation of Glycerol at Acid and Basic Conditions" Catalysts 11, no. 1: 94. https://doi.org/10.3390/catal11010094
APA StyleKaminski, P. (2021). The Application of Copper-Gold Catalysts in the Selective Oxidation of Glycerol at Acid and Basic Conditions. Catalysts, 11(1), 94. https://doi.org/10.3390/catal11010094