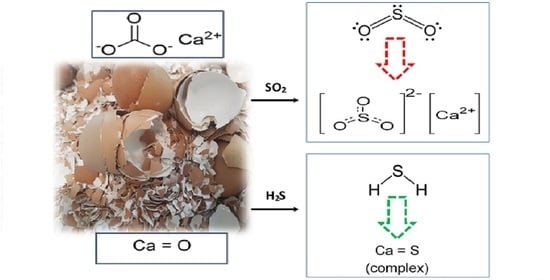
Valorization of Raw and Calcined Chicken Eggshell for Sulfur Dioxide and Hydrogen Sulfide Removal at Low Temperature
Abstract
:1. Introduction
2. Results and Discussions
2.1. Characterization of Raw and Calcined Eggshell before and after Adsorption Tests
2.2. Effect of the Eggshell Membrane and Particle Size
2.3. Effect of Calcination Temperature
2.4. Effect of Reaction Temperature and Humidity
2.5. Comparison Study
3. Materials and Methods
3.1. Sorbent Preparation
3.2. Adsorption Tests
3.3. Process Parameters Study
3.4. Characterization of the Sorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Du, E.; Dong, D.; Zeng, X.; Sun, Z.; Jiang, X.; de Vries, W. Direct Effect of Acid Rain on Leaf Chlorophyll Content of Terrestrial Plants in China. Sci. Total Environ. 2017, 605, 764–769. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Yang, H.; Zhang, H.; Zhang, X. Experimental Study on SO2 Recovery Using a Sodium—Zinc Sorbent Based Flue Gas Desulfurization Technology. Chin. J. Chem. Eng. 2015, 23, 241–246. [Google Scholar] [CrossRef]
- Córdoba, P. Status of Flue Gas Desulphurisation (FGD) Systems from Coal-Fired Power Plants: Overview of the Physic-Chemical Control Processes of Wet Limestone FGDs. Fuel 2015, 144, 274–286. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, F.; Liu, X.; Gao, J.; Qie, Z.; Zhao, G. The Effect of Nitrogen-Containing Functional Groups on SO2 Adsorption on Carbon Surface: Enhanced Physical Adsorption Interactions. Surf. Sci. 2018, 677, 78–82. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Allinson, G.; Li, X.; Stagnitti, F.; Murray, F.; Xiong, X. Effects of Sulfur Dioxide Pollution on the Translocation and Accumulation of Heavy Metals in Soybean Grain. Environ. Sci. Pollut. Res. 2011, 18, 1090–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Sheng, Y.; Sun, X. Simultaneous Removal of SO2 and NOX by a Combination of Red Mud and Coal Mine Drainage. Environ. Eng. Sci. 2019, 36, 444–452. [Google Scholar] [CrossRef]
- Silas, K.; Ghani, W.A.; Choong, T.S.; Rashid, U. Carbonaceous Materials Modified Catalysts for Simultaneous SO2/NOx Removal from Flue Gas: A Review. Catal. Rev. 2019, 61, 134–161. [Google Scholar] [CrossRef]
- El Asri, O.; Hafidi, I.; elamin Afilal, M. Comparison of Biogas Purification by Different Substrates and Construction of a Biogas Purification System. Waste Biomass Valorization 2015, 6, 459–464. [Google Scholar] [CrossRef]
- Ahmad, W.; Sethupathi, S.; Kanadasan, G.; Lau, L.C.; Kanthasamy, R. A Review on the Removal of Hydrogen Sulfide from Biogas by Adsorption Using Sorbents Derived from Waste. Rev. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen Sulfide Capture: From Absorption in Polar Liquids to Oxide, Zeolite, and Metal-Organic Framework Adsorbents and Membranes. Chem. Rev. 2017, 117, 14. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A Review of Biogas Purification Processes. Biofuels Bioprod. Biorefining 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Baláž, M. Ball Milling of Eggshell Waste as a Green and Sustainable Approach: A Review. Adv. Colloid Interface Sci. 2018, 256, 256–275. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Benelli, P.; Amante, E.R. A Literature Review on Adding Value to Solid Residues: Egg Shells. J. Clean. Prod. 2013, 46, 42–47. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO); U.N. Livestock Production. 2015. Available online: http://www.fao.org/docrep/005/y4252e/y4252e07.htm (accessed on 30 August 2019).
- Rohim, R.; Ahmad, R.; Ibrahim, N.; Hamidin, N.; Abidin, C.Z.A. Characterization of Calcium Oxide Catalyst from Eggshell Waste. Adv. Environ. Biol. 2014, 8, 35–38. [Google Scholar]
- De Angelis, G.; Medeghini, L.; Conte, A.M.; Mignardi, S. Recycling of Eggshell Waste into Low-Cost Adsorbent for Ni Removal from Wastewater. J. Clean. Prod. 2017, 164, 1497–1506. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Eggshell Waste as Catalyst: A Review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Eghbali Babadi, F.; Masoudi Soltani, S.; Aroua, M.K.; Babamohammadi, S.; Mousavi Moghadam, A. Carbon Dioxide Adsorption on Nitrogen-Enriched Gel Beads from Calcined Eggshell/Sodium Alginate Natural Composite. Process Saf. Environ. Prot. 2017, 109, 387–399. [Google Scholar] [CrossRef]
- Sethupathi, S.; Kai, Y.C.; Kong, L.L.; Munusamy, Y.; Bashir, M.J.K.; Iberahim, N. Preliminary Study of Sulfur Dioxide Removal Using Calcined Egg Shell. Malays. J. Anal. Sci. 2017, 21, 719–725. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, L. Hybrid Adsorbent Prepared from Renewable Lignin and Waste Egg Shell for SO2 Removal: Characterization and Process Optimization. Ecol. Eng. 2018, 115, 139–148. [Google Scholar] [CrossRef]
- Cho, Y.B.; Seo, G.; Chang, D.R. Transesterification of Tributyrin with Methanol over Calcium Oxide Catalysts Prepared from Various Precursors. Fuel Process. Technol. 2009, 90, 1252–1258. [Google Scholar] [CrossRef]
- Witoon, T. Characterization of Calcium Oxide Derived from Waste Eggshell and Its Application as CO2 Sorbent. Ceram. Int. 2011, 37, 3291–3298. [Google Scholar] [CrossRef]
- Gergely, G.; Weber, F.; Lukacs, I.; Toth, A.L.; Horvath, Z.E.; Mihaly, J.; Balazsi, C. Preparation and Characterization of Hydroxyapatite from Eggshell. Ceram. Int. 2010, 36, 803–806. [Google Scholar] [CrossRef]
- Engin, B.; Demirtaş, H.; Eken, M. Temperature Effects on Egg Shells Investigated by XRD, IR and ESR Techniques. Radiat. Phys. Chem. 2006, 75, 268–277. [Google Scholar] [CrossRef]
- Lee, K.T.; Mohamed, A.R.; Bhatia, S.; Chu, K.H. Removal of Sulfur Dioxide by Fly Ash/CaO/CaSO4 Sorbents. Chem. Eng. J. 2005, 114, 171–177. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Y.; Chen, C. Simultaneous Removal of SO2 and NO from Flue Gas Using Multicomposite Active Absorbent. Ind. Eng. Chem. Res. 2011, 51, 480–486. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and Adsorption Properties of Eggshells and Eggshell Membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Al-ghouti, M.A.; Khan, M. Eggshell Membrane as a Novel Bio Sorbent for Remediation of Boron from Desalinated Water. J. Environ. Manag. 2017, 207, 405–416. [Google Scholar] [CrossRef]
- Pramanpol, N.; Nitayapat, N. Adsorption of Reactive Dye by Eggshell and Its Membrane. Kasetsart J. 2006, 40, 192–197. [Google Scholar]
- Al-Hosney, H.A.; Grassian, V.H. Water, Sulfur Dioxide and Nitric Acid Adsorption on Calcium Carbonate: A Transmission and ATR-FTIR Study. Phys. Chem. Chem. Phys. 2005, 7, 1266–1276. [Google Scholar] [CrossRef]
- De-Diego, L.; Abad, A.; Garcia-Lebiano, F.; Adanez, J.; Gayan, P. Simultaneous Calcination and Sulfidation of Calcium-Based Sorbents. Ind. Eng. Chem. Res. 2004, 43, 3261–3269. [Google Scholar] [CrossRef]
- Valverde, J.M.; Medina, S. Reduction of Calcination Temperature in the Calcium Looping Process for CO2 Capture by Using Helium: In Situ XRD Analysis. Sustain. Chem. Eng. 2016, 4, 7090–7097. [Google Scholar] [CrossRef]
- Doǧu, T.I. The Importance of Pore Structure and Diffusion in the Kinetics of Gas-Solid Non-Catalytic Reactions: Reaction of Calcined Limestone with SO2. Chem. Eng. J. 1981, 21, 213–222. [Google Scholar] [CrossRef]
- Quina, M.J.; Soares, M.A.R.; Quinta-ferreira, R. Applications of Industrial Eggshell as a Valuable Anthropogenic Resource. Resour. Conserv. Recycl. 2017, 123, 176–186. [Google Scholar] [CrossRef]
- Hartman, M.; Svoboda, K.; Trnka, O.; Čermák, J. Reaction between Hydrogen Sulfide and Limestone Calcines. Ind. Eng. Chem. Res. 2002, 41, 2392–2398. [Google Scholar] [CrossRef]
- Borgwardt, R.H. Sintering of Nascent Calcium Oxide. Chem. Eng. Sci. 1989, 44, 53–60. [Google Scholar] [CrossRef]
- Slack, A.V.; Falkenberry, H.L.; Harrington, R.E. Sulfur Oxide Removal from Waste Gases: Lime-Limestone Scrubbing Technology. J. Air Pollut. Control Assoc. 1972, 22, 159–166. [Google Scholar] [CrossRef]
- Liu, C.-F.; Shih, S.-M.; Lin, R.-B. Kinetics of the Reaction of Ca(OH)2/Fly Ash Sorbent with SO2 at Low Temperatures. Chem. Eng. Sci. 2002, 57, 93–104. [Google Scholar] [CrossRef]
- Agnihotri, R.; Chauk, S.S.; Mahuli, S.K.; Fan, L.S. Mechanism of CaO Reaction with H2S: Diffusion through CaS Product Layer. Chem. Eng. Sci. 1999, 54, 3443–3453. [Google Scholar] [CrossRef]
- Krammer, G.; Brunner, C.; Khinast, J.; Staudinger, G. Reaction of Ca(OH)2 with SO2 at Low Temperature. Ind. Eng. Chem. Res. 1997, 36, 1410–1418. [Google Scholar] [CrossRef]
- Galloway, B.D.; MacDonald, R.A.; Padak, B. Characterization of Sulfur Products on CaO at High Temperatures for Air and Oxy-Combustion. Int. J. Coal Geol. 2016, 167, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Pan, W.; Srimat, S.T.; Zheng, J.; Li, Y.; Wang, Y.H.; Xu, B.Q.; Zhu, Q.M. Tri-Reforming of Methane over Ni Catalysts for CO2 Conversion to Syngas with Desired H2CO Ratios Using Flue Gas of Power Plants without CO2 Separation. Stud. Surf. Sci. Catal. 2004, 153, 315–322. [Google Scholar]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xu, X. Study of the Effect of Fly Ash on Desulfurization by Lime. Fuel 2001, 80, 1969–1973. [Google Scholar] [CrossRef]
- Fenouil, L.A.; Towler, G.P.; Lynn, S. Removal of H2S from Coal Gas Using Limestone: Kinetic Considerations. Ind. Eng. Chem. Res. 1994, 33, 265–272. [Google Scholar] [CrossRef]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-Pressure Solubility of Gases in Liquid Water. Chem. Rev. 1977, 77, 219–262. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Usher, C.R.; Grassian, V.H. Reactions of Sulfur Dioxide on Calcium Carbonate Single Crystal and Particle Surfaces at the Adsorbed Water Carbonate Interface. Phys. Chem. Chem. Phys. 2007, 9, 3011. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.G.; Minh, D.P.; Nzihou, A.; Sharrock, P. Valorization of Calcium Carbonate-Based Solid Wastes for the Treatment of Hydrogen Sulfide in a Semi-Continuous Reactor. Chem. Eng. J. 2019, 360, 1167–1176. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Yao, J.; Gao, L.; Ma, X.; Zhao, Y. Experimental Study on Removals of SO2 and NOX Using Adsorption of Activated Carbon/Microwave Desorption. J. Air Waste Manag. Assoc. 2012, 62, 1012–1021. [Google Scholar] [CrossRef]
- Garcia-Labiano, F.; De Diego, L.F.; Adánez, J. Effectiveness of Natural, Commercial, and Modified Calcium-Based Sorbents as H2S Removal Agents at High Temperatures. Environ. Sci. Technol. 1999, 33, 288–293. [Google Scholar] [CrossRef]
- Adanez, J.; Fierro, V.; Garcia-Labiano, F.; Palacios, J. Study of Modified Calcium Hydroxides for Enhancing SO2 Removal during Sorbent Injection in Pulverized Coal Boilers. Fuel 1997, 76, 257–265. [Google Scholar] [CrossRef]
- Dahlan, I.; Mohamed, A.R.; Kamaruddin, A.H.; Lee, K.T. Dry SO2 Removal Process Using Calcium/Siliceous-Based Sorbents: Deactivation Kinetics Based on Breakthrough Curves. Chem. Eng. Technol. Ind. Chem. Equip. Process Eng. 2007, 30, 663–666. [Google Scholar] [CrossRef]
- Jung, J.; Yoo, K.; Kim, H.; Lee, H.; Shon, B.-H. Reuse of Waste Oyster Shells as a SO2/NOx Removal Absorbent. J. Ind. Eng. Chem. 2007, 13, 512–517. [Google Scholar]
- Nelli, C.H.; Rochelle, G.T. Simultaneous Sulfur Dioxide and Nitrogen Dioxide Removal by Calcium Hydroxide and Calcium Silicate Solids. J. Air Waste Manag. Assoc. 1998, 48, 819–828. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, H.; Wang, S.; Zhuo, Y.; Chen, C.; Xu, X. Simultaneous Removal of SO2 and NO from Flue Gas with Calcium-Based Sorbent at Low Temperature. Ind. Eng. Chem. Res. 2006, 45, 6099–6103. [Google Scholar] [CrossRef]
- Ghorishi, S.B.; Singer, C.F.; Jozewicz, W.S.; Sedman, C.B.; Srivastava, R.K. Simultaneous Control of Hg0, SO2, and NOx by Novel Oxidized Calcium-Based Sorbents. J. Air Waste Manag. Assoc. 2002, 52, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iberahim, N.; Sethupathi, S.; Bashir, M.J.K. Optimization of Palm Oil Mill Sludge Biochar Preparation for Sulfur Dioxide Removal. Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef]









| Calcination Temperature (°C) | BET Surface Area (m2/g) |
|---|---|
| 800 | 2.98 |
| 900 | 6.74 |
| 950 | 6.54 |
| 1000 | 6.30 |
| 1100 | 2.68 |
| Sample | EDX Figure | Elements | Percentage |
|---|---|---|---|
| RES |  | Ca | 16.85 |
| C | 31.80 | ||
| O | 49.13 | ||
| Others | 2.22 | ||
| RES Spent SO2 |  | Ca | 12.08 |
| C | 27.68 | ||
| O | 57.84 | ||
| S | 0.55 | ||
| Others | 1.85 | ||
| RES Spent H2S |  | Ca | 11.35 |
| C | 56.25 | ||
| O | 30.95 | ||
| S | 0.11 | ||
| Others | 1.34 | ||
| (CES 900 °C) |  | Ca | 24.93 |
| C | 35.87 | ||
| O | 38.23 | ||
| Others | 0.47 | ||
| (CES 900 °C) Spent SO2 |  | Ca | 14.65 |
| C | 41.33 | ||
| O | 42.4 | ||
| S | 0.65 | ||
| Others | 1.06 | ||
| (CES 900 °C) Spent H2S |  | Ca | 16.05 |
| C | 36.8 | ||
| O | 46.25 | ||
| S | 0.21 | ||
| Others | 0.69 |
| Temperature (°C) | Proximate Analysis | RES (%) | CES (%) |
|---|---|---|---|
| 25–120 | Moisture | 1.03 | 0.28 |
| 120–450 | Volatile content | 4.18 | 8.01 |
| 450–800 | CO2 | 43.17 | 1.63 |
| 800–900 | Residue (CaO) | 51.62 | 90.08 |
| Sample | Particle Size (µm) | Saturation Time (min) | Adsorption Capacity (mg/g) | Original pH | pH (after Adsorption) | |||
|---|---|---|---|---|---|---|---|---|
| SO2 | H2S | SO2 | H2S | SO2 | H2S | |||
| Raw eggshell with membrane | <90 | 32.2 | 44.0 | 1.09 | 0.65 | 9.2 ± 0.2 | 8.7 ± 0.2 | 8.9 ± 0.2 |
| 90–125 | 29.0 | 20.5 | 0.89 | 0.19 | ||||
| 125–180 | 15.3 | 8.0 | 0.61 | 0.08 | ||||
| Raw eggshell without membrane | <90 | 29.8 | 33.0 | 0.98 | 0.26 | 8.9 ± 0.2 | 8.4 ± 0.2 | 8.6 ± 0.2 |
| 90–125 | 25.5 | 17.5 | 0.66 | 0.14 | ||||
| 125–180 | 13.3 | 7.5 | 0.54 | 0.05 | ||||
| Calcination Temperature (°C) | BET Surface Area (m2/g) | Adsorption Capacity (mg/g) | Original pH | pH (After Adsorption) | ||
|---|---|---|---|---|---|---|
| SO2 | H2S | SO2 | H2S | |||
| 800 | 2.98 | 2.12 | 1.30 | 10.9 ± 0.2 | 10.1 ± 0.2 | 10.3 ± 0.2 |
| 900 | 6.74 | 3.53 | 2.62 | 11.8 ± 0.2 | 10.9 ± 0.2 | 11.1 ± 0.2 |
| 950 | 6.54 | 3.22 | 2.35 | 12.0 ± 0.2 | 11.2 ± 0.2 | 11.4 ± 0.2 |
| 1000 | 6.30 | 2.69 | 1.85 | 12.1 ± 0.2 | 11.3 ± 0.2 | 11.5 ± 0.2 |
| 1100 | 2.68 | 2.55 | 1.66 | 12.3 ± 0.2 | 11.4 ± 0.2 | 11.5 ± 0.2 |
| Reaction Condition | Raw Eggshell (mg/g) | Calcined Eggshell (900 °C) (mg/g) | ||
|---|---|---|---|---|
| SO2 | H2S | SO2 | H2S | |
| Reaction Temperature (100 °C) | 1.43 | 0.77 | 3.93 | 3.37 |
| Reaction Temperature (200 °C) | 2.03 | 1.20 | 4.30 | 4.22 |
| Humidity (40%) | 8.89 | 1.42 | 11.68 | 7.96 |
| Gas | Sorbent | Conditions | Adsorption Capacity/ Removal Efficiency | Ref. |
|---|---|---|---|---|
| SO2 | Ethanol treated calcined limestone (CaO) (BET = 35.5 m2/g) | Reaction temperature = 1050 °C SO2—2000 ppm by volume Residence time = 1 s Ca/S ratio = 3 | Around 55% | [52] |
| Commercial Ca(OH)2 mixed with rice husk ash (BET = 106.10 m2/g) | Reaction temperature—100 °C SO2 = 1000 ppm NO =500 ppm CO2 = 12% N2 = balance | 10.72 mg/g | [53] | |
| Commercial Ca(OH)2 mixed oil palm ash (BET = 88.3 m2/g) | 5.36 mg/g | |||
| Commercial Ca(OH)2 mixed with coal fly ash (BET = 62.2 m2/g) | 4.29 mg/g | |||
| Ca(OH)2 obtained from oyster shell (BET = 12.9 m2/g)—CaO content = 53.8% | Reaction temperature—150 °C) SO2 = 1800 ppm NOx = 250 ppm O2 = 6% Water vapors = 10% | 0.78 mmol/g | [54] | |
| Hydrated Lime (BET = 8.76 m2/g) | Reaction temperature = 70 °C NO2 = 249 ppm SO2 = 906 ppm Relative humidity = 60% | 25% (SO2) | [55] | |
| Ca(OH)2 mixed with fly ash in ratio of 1:3 with additional treatment with KMnO4 (BET = 19.04 m2/g) | Reaction temperature = 80 °C SO2 = 500 ppm NO = 200 ppm O2 = 5% Relative Humidity = 80% | 60–80% | [56] | |
| Oxidant enriched Ca(OH)2 (BET = 11 to 14 m2/g) | Reaction temperature—80 °C SO2—600 ppm NO—300 ppm O2—8% H2O—10.5% | 80–100 mg/g | [57] | |
| Calcined eggshell (BET = 6.74 m2/g) | Reaction temperature—30 °C SO2—300 ppm N2-balance Relative humidity—40% | 11.68 mg/g | This work | |
| H2S | CaO from waste CaCO3 (BET < 3 m2/g) | Reaction temperature—20 °C H2S—50 ppm in air TGA Analysis Reaction time 55 min | 55% | [10] |
| CaCO3 from waste (BET < 3.98 m2/g) | Reaction temperature-Ambient Biogas with 200 ppmv H2S Reaction time 400 min | 85% | ||
| Dried fly ash (BET = 17.71 m2/g) | Reaction temperature-Not Given H2S—300 ppm in air | 10.4 mg/g | ||
| Australian red soil (BET = not given) | Reaction temperature-Not Given H2S = 2000 ppm N2-balance | 18.8 mg/g | ||
| Calcined eggshell (BET 6.74 m2/g) | Reaction temperature—30 °C H2S—300 ppm N2-balance Relative humidity—40% | 7.96 mg/g | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Sethupathi, S.; Munusamy, Y.; Kanthasamy, R. Valorization of Raw and Calcined Chicken Eggshell for Sulfur Dioxide and Hydrogen Sulfide Removal at Low Temperature. Catalysts 2021, 11, 295. https://doi.org/10.3390/catal11020295
Ahmad W, Sethupathi S, Munusamy Y, Kanthasamy R. Valorization of Raw and Calcined Chicken Eggshell for Sulfur Dioxide and Hydrogen Sulfide Removal at Low Temperature. Catalysts. 2021; 11(2):295. https://doi.org/10.3390/catal11020295
Chicago/Turabian StyleAhmad, Waseem, Sumathi Sethupathi, Yamuna Munusamy, and Ramesh Kanthasamy. 2021. "Valorization of Raw and Calcined Chicken Eggshell for Sulfur Dioxide and Hydrogen Sulfide Removal at Low Temperature" Catalysts 11, no. 2: 295. https://doi.org/10.3390/catal11020295
APA StyleAhmad, W., Sethupathi, S., Munusamy, Y., & Kanthasamy, R. (2021). Valorization of Raw and Calcined Chicken Eggshell for Sulfur Dioxide and Hydrogen Sulfide Removal at Low Temperature. Catalysts, 11(2), 295. https://doi.org/10.3390/catal11020295