Hydrogen Production via Pd-TiO2 Photocatalytic Water Splitting under Near-UV and Visible Light: Analysis of the Reaction Mechanism
Abstract
:1. Introduction
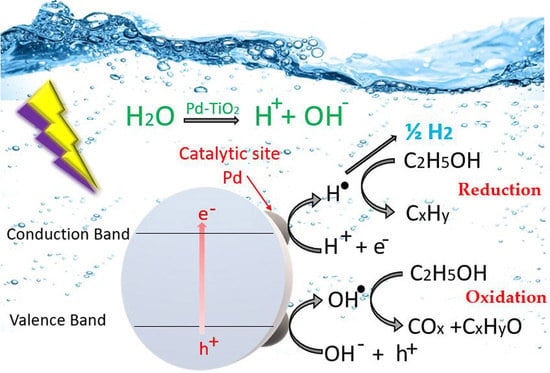
2. Proposed Reaction Mechanism
- (a)
- Hydrogen production proceeds via a ‘‘series–parallel’’ redox reaction network.
- (b)
- Water splits, forming intermediate OH• and H• radicals, with H• reacting further and yielding molecular hydrogen, as shown in Equations (1) and (2).
- (c)
- Ethanol, as an OH· organic scavenger, is consumed via different reaction pathways to form various oxidation by-products, such as acetaldehyde, CO, and CO2, as described with Equations (3)–(7).
- (d)
- Ethanol and ethanol by-products are reduced via the H· radicals present, yielding methane, ethane, and ethylene, as reported with Equations (8)–(10).
2.1. Step 1: Hydrogen Production Pathway

2.2. Step 2: Ethanol Derived By-Products Formation
2.2.1. Oxidation Reactions
- (a)
- Acetaldehyde
- (b)
- Carbon Dioxide
- (c)
- Carbon Monoxide
2.2.2. Reduction Reactions
- (d)
- Methane
- (e)
- Ethane
- (f)
- Ethylene
2.3. Step 3: Ethanol Photoregeneration
- CO molecules are strongly adsorbed onto a Pd-TiO2 surface until a second CO is available for C-C coupling.
- Due to the reduced band gap of the photocatalyst (2.51 eV), electrons jump from the valence band to the conduction band and are trapped by the palladium.
- The photogenerated electrons are used to activate and reduce the CO, which lead to ethanol formation via hydrogenation.
- (a)
- C-C coupling involves electron transfer, with this leading to the formation of the *C2O2-intermediate.
- (b)
- Once the *C2O2-intermediate is generated, hydrogenation, electron transfer takes place, with the *C2O2H yielding to ethanol.
3. Results and Discussion
3.1. Photocatalytic Hydrogen Production under Near-UV Light and Visible Light
3.2. By-Products Formation
3.3. H• and OH• Radical Group Balance
- The H• radicals formed can be calculated via the accounting of the experimentally obtained hydrogen, as postulated in Equation (2), and via the hydrogen consumption required by the synthesis of various reduced products (methane, ethane), as given by Equations (8) and (9).
- The OH• can be quantified by considering the OH• radicals consumed, according to their stochiometric requirements from several oxidation reactions, as given by Equations (3)–(6) and (11). Thus, the OH• consumption should account for acetaldehyde, carbon monoxide, carbon dioxide, and hydrogen peroxide species.
3.3.1. Further Establishing of the Total OH• Formed during Photocatalytic Hydrogen Production
3.3.2. pH Influence on the Photocatalytic Reaction
- (a)
- H2 is a main product from the photocatalytic water splitting reaction using ethanol as an organic scavenger and a 0.25 wt.% Pd-TiO2-nUV or alternatively, a 0.25 wt.% Pd-TiO2-VIS.
- (b)
- Formed photoreduction species (methane, ethane) and photooxidation species (CO2, acetaldehyde) are all important carbon containing by-products.
- (c)
- Hydrogen peroxide, present in the liquid phase, is formed and adsorbed on the photocatalyst during water splitting.
- (d)
- OH− species in the water solution progressively increase with irradiation time, with this leading to a pH increase.
4. Experimental Methodology
4.1. Photocatalyst Synthesis
4.2. Photocatalyst Characterization
4.3. Photo-CREC Water-II Reactor
4.4. Photocatalytic Experiments
4.5. Analytical Techniques
4.5.1. Determination of H2O2 Concentrations
4.5.2. Effect of the pH on the Photocatalytic Reaction
4.6. Adsorption of Hydrogen Peroxide and Ethanol
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Ce | Concentration in the liquid of adsorbate at equilibrium |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CH4 | Methane |
| C2H6 | Ethane |
| C2H4 | Ethylene |
| C2H4O | Acetaldehyde |
| Dp | Pore diameter (cm) |
| e− | Electron |
| h+ | Hole |
| F-127 | Poly (ethylene oxide)/poly (propylene oxide)/poly (ethylene oxide) |
| H• | Hydrogen radical |
| H2O | Water |
| H2O2 | Hydrogen Peroxide |
| K | Adsorption constant |
| OH− | Hydroxide ions |
| OH• | Hydroxide radicals |
| Pd | Palladium |
| PdCl2 | Palladium II chloride |
| PEO | Poly (ethylene oxide) |
| PPO | Poly (propylene oxide) |
| Qe | Equilibrium adsorbent-phase concentration |
| Qe,max | Maximum adsorption capacity |
| t | Time (h) |
| TiO2 | Titanium dioxide |
| Acronyms | |
| BLB | Black Light Blue Lamp |
| BET | Brunauer–Emmett–Teller Surface Area Method |
| CB | Conduction Band |
| DP25 | Degussa P25 (TiO2) |
| DPD | N, N-dimethyl-p-phenylenediamine |
| EISA | Evaporation-Induced-Self-Assembly |
| FID | Flame Ionization Detector |
| GC | Gas Chromatography |
| HPLC | High Performance Liquid Chromatography |
| MIEB | Macroscopic Irradiation Energy Balance |
| PCW-II | Photo-CREC Water-II Reactor |
| PC | Photocatalyst Concentration |
| Pd-TiO2-nUV | Palladium doped Mesoporous TiO2 under Near-UV light |
| Pd-TiO2-VIS | Palladium doped Mesoporous TiO2 after 1 h under Near UV light and 5 h under Visible Light |
| pH | Potential of Hydrogen |
| STP | Standard Temperature and Pressure (273 K and 1 atm) |
| TPR | Temperature Programmed Reduction |
| TCD | Thermal Conductivity Detector |
| UV | Ultraviolet |
| VB | Valence Band |
| VIS | Visible light |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
Appendix A. Carbon Containing Species Balance
- Moles of carbon at t = 0 h in the liquid phase:
- Moles of carbon at t = 6 h in the liquid phase:
- Ethanol in the gas phase:
- Methane in the gas phase:
- Ethane in the gas phase:
- Ethylene in the gas phase:
- Acetaldehyde in the gas phase:
- Carbon monoxide in the gas phase:
- Carbon dioxide in the gas phase:
| Time (h) | Concentration (M) |
|---|---|
| 0 | 0.34171 |
| 1 | 0.33529 |
| 2 | 0.33714 |
| 3 | 0.34312 |
| 4 | 0.33742 |
| 5 | 0.33960 |
| 6 | 0.34110 |
Appendix B. H• and OH• Radicals Balance
Appendix C. Detection of H2 and Carbon Containing Species by a Shimadzu CG 2010
| Rate | Temperature (°C) | Hold Time (min) |
| - | 50 | 4 |
| 20.0 | 200 | 18.5 |



References
- Chang, T.; Chu, H.-P.; Chen, W.-Y. Energy consumption and economic growth in 12 Asian countries: Panel data analysis. Appl. Econ. Lett. 2013, 20, 282–287. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Sugihara, H. Development of New Photocatalytic Water Splitting into H2 and O2 using Two Different Semiconductor Photocatalysts and a Shuttle Redox Mediator IO3-/I-. J. Phys. Chem. B 2005, 109, 16052–16061. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.H.; Larsen, P.H.; Mogensen, M.B. Hydrogen and synthetic fuel production from renewable energy sources. Int. J. Hydrogen Energy 2007, 32, 3253–3257. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Ipsakis, D.; Voutetakis, S.; Seferlis, P.; Stergiopoulos, F.; Elmasides, C. Power management strategies for a stand-alone power system using renewable energy sources and hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 7081–7095. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.-M.; Lu, G.Q. (Max) Titania-based photocatalysts—Crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Arakawa, H. Significant effect of iodide addition on water splitting into H2 and O2 over Pt-loaded TiO2 photocatalyst: Suppression of backward reaction. Chem. Phys. Lett. 2003, 371, 360–364. [Google Scholar] [CrossRef]
- Mills, A.; Porter, G. Photosensitised dissociation of water using dispersed suspensions of n-type semiconductors. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1982, 78, 3659–3669. [Google Scholar] [CrossRef]
- López, C.R.; Melián, E.P.; Méndez, J.O.; Santiago, D.E.; Rodríguez, J.D.; Díaz, O.G. Comparative study of alcohols as sacrificial agents in H2 production by heterogeneous photocatalysis using Pt/TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2015, 312, 45–54. [Google Scholar] [CrossRef]
- Galińska, A.; Walendziewski, J. Photocatalytic Water Splitting over Pt-TiO2 in the Presence of Sacrificial Reagents. Energy Fuels 2005, 19, 1143–1147. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic Activity Enhanced by Plasmonic Resonant Energy Transfer from Metal to Semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef] [PubMed]
- Rusinque, B.; Escobedo, S.; De Lasa, H. Photocatalytic Hydrogen Production Under Near-UV Using Pd-Doped Mesoporous TiO2 and Ethanol as Organic Scavenger. Catalysts 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Rusinque, B.; Escobedo, S.; De Lasa, H. Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light. Catalysts 2020, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- Holloway, P.; McGuire, G. Handbook of Compound Semiconductor; Noyes Publications: Park Ridge, NJ, USA, 1995. [Google Scholar]
- Escobedo, S.; Serrano, B.; Calzada, A.; Moreira, J.; De Lasa, H. Hydrogen production using a platinum modified TiO2 photocatalyst and an organic scavenger. Kinetic modeling. Fuel 2016, 181, 438–449. [Google Scholar] [CrossRef]
- Rusinque, B. Hydrogen Production by Photocatalytic Water Splitting under Near-UV and Visible Light Using Doped Pt and Pd TiO2. Master’s Thesis, The University of Western Ontario, London, ON, Canada, September 2018. [Google Scholar]
- Bai, S.; Shao, Q.; Wang, P.; Dai, Q.; Wang, X.; Huang, X. Highly Active and Selective Hydrogenation of CO2 to Ethanol by Ordered Pd–Cu Nanoparticles. J. Am. Chem. Soc. 2017, 139, 6827–6830. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Wei, W.; Sun, Y. Advances in Clean Fuel Ethanol Production from Electro-, Photo- and Photoelectro-Catalytic CO2 Reduction. Catalysts 2020, 10, 1287. [Google Scholar] [CrossRef]
- Arrouvel, C.; Digne, M.; Breysse, M.; Toulhoat, H.; Raybaud, P. Effects of morphology on surface hydroxyl concentration: A DFT comparison of anatase-TiO2 and γ-alumina catalytic supports. J. Catal. 2004, 222, 152–166. [Google Scholar] [CrossRef]
- Moreira, J.; Serrano, B.; Ortiz, A.; De Lasa, H. A unified kinetic model for phenol photocatalytic degradation over TiO2 photocatalysts. Chem. Eng. Sci. 2012, 78, 186–203. [Google Scholar] [CrossRef]
- Sahel, K.; Elsellami, L.; MirAli, I.; Dappozze, F.; Bouhent, M.; Guillard, C. Hydrogen peroxide and photocatalysis. Appl. Catal. B Environ. 2016, 188, 106–112. [Google Scholar] [CrossRef]
- Enzweiler, H.; Yassue-Cordeiro, P.H.; Schwaab, M.; Barbosa-Coutinho, E.; Scaliante, M.H.N.O.; Fernandes, N.R.C. Catalyst concentration, ethanol content and initial pH effects on hydrogen production by photocatalytic water splitting. J. Photochem. Photobiol. A Chem. 2020, 388, 112051. [Google Scholar] [CrossRef]
- Salas, S.E.; de Lasa, S.H. Photocatalytic Water Splitting Using a Modified Pt-TiO2. Kinetic Modeling and Hydrogen Production Efficiency. Master’s Thesis, The University of Western Ontario, London, ON, Canada, August 2013. [Google Scholar]
- Kosmulski, M. The significance of the difference in the point of zero charge between rutile and anatase. Adv. Colloid Interface Sci. 2002, 99, 255–264. [Google Scholar] [CrossRef]
- Arlos, M.J.; Hatat-Fraile, M.M.; Liang, R.; Bragg, L.M.; Zhou, N.Y.; Andrews, S.A.; Servos, M.R. Photocatalytic decomposition of organic micropollutants using immobilized TiO2 having different isoelectric points. Water Res. 2016, 101, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Parida, K.; Sant, B. Studies on MnO2—III. The kinetics and the mechanism for the catalytic decomposition of H2O2 over different crystalline modifications of MnO2. Electrochim. Acta 1981, 26, 1157–1167. [Google Scholar] [CrossRef]
- Domínguez-Henao, L.; Turolla, A.; Monticelli, D.; Antonelli, M. Assessment of a colorimetric method for the measurement of low concentrations of peracetic acid and hydrogen peroxide in water. Talanta 2018, 183, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, X.-B.; Ding, L.; Luo, S.-L. Application of nanotechnology in the removal of heavy metal from water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2018; pp. 83–147. [Google Scholar]














| μmoles of H• Formed Equations (2), (8) and (9) (a) | μmoles of OH• Consumed Equations (3)–(6) (b) | |
|---|---|---|
| Pd-TiO2-nUV. | 10,191.5 | 2169.6 |
| Pd-TiO2-VIS | 2620.3 | 2342.4 |
| μmoles of H• Formed as Shown in Equations (2), (8) and (9) | Cumulative μmoles of OH• Consumed Forming H2O2 as Shown in Equation (11) (Liquid Phase) | Cumulative μmoles of OH• Consumed as Shown in Equations (3)–(7) and (11) | |
|---|---|---|---|
| Pd-TiO2-nUV. | 10,191.5 | 188.4 | 2359 |
| Pd-TiO2-VIS | 2620.3 | 89.1 | 2431 |
| Adsorption Constants | ||
|---|---|---|
| K | Qe,max | |
| Hydrogen Peroxide | 0.93 mg−1 L | 11.1 mg−1 gcat |
| Cumulative μmoles of H• Formed Calculated with Equations (2), (8) and (9) | Cumulative μmoles of OH• Forming H2O2 (Adsorbed) | Cumulative μmoles of OH• Consumed Using Equations (3)–(7) and (11), and OH• Adsorbed | |
|---|---|---|---|
| Pd-TiO2-nUV | 10,191 | 84.4 | 2444 |
| Pd-TiO2-VIS | 2620 | 40.1 | 2472 |
| Cumulative μmoles of H• Formed via Equation (2), (8) and (9) | Cumulative μmoles of OH• Produced via pH Change | Cumulative μmoles of OH• Consumed Via Equations (3), (6), and (11) Based on H2O2 Adsorbed and pHChange | Percentual H• OH• Balance Closure (%) | |
|---|---|---|---|---|
| Pd-TiO2-UV | 10,191 | 7662.5 | 10,106.3 | 99.2 |
| Pd-TiO2-VIS | 2620 | 81.9 | 2553.5 | 97.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinque, B.; Escobedo, S.; de Lasa, H. Hydrogen Production via Pd-TiO2 Photocatalytic Water Splitting under Near-UV and Visible Light: Analysis of the Reaction Mechanism. Catalysts 2021, 11, 405. https://doi.org/10.3390/catal11030405
Rusinque B, Escobedo S, de Lasa H. Hydrogen Production via Pd-TiO2 Photocatalytic Water Splitting under Near-UV and Visible Light: Analysis of the Reaction Mechanism. Catalysts. 2021; 11(3):405. https://doi.org/10.3390/catal11030405
Chicago/Turabian StyleRusinque, Bianca, Salvador Escobedo, and Hugo de Lasa. 2021. "Hydrogen Production via Pd-TiO2 Photocatalytic Water Splitting under Near-UV and Visible Light: Analysis of the Reaction Mechanism" Catalysts 11, no. 3: 405. https://doi.org/10.3390/catal11030405
APA StyleRusinque, B., Escobedo, S., & de Lasa, H. (2021). Hydrogen Production via Pd-TiO2 Photocatalytic Water Splitting under Near-UV and Visible Light: Analysis of the Reaction Mechanism. Catalysts, 11(3), 405. https://doi.org/10.3390/catal11030405