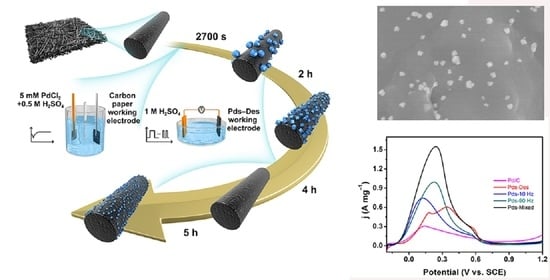
Palladium Particles Modified by Mixed-Frequency Square-Wave Potential Treatment to Enhance Electrocatalytic Performance for Formic Acid Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations Results
2.2. Electrochemical Results
3. Materials and Methods
3.1. Reagents and Materials
3.2. Reagents and Materials
3.3. Reagents and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larsen, R.; Ha, S.; Zakzeski, J.; Masel, R.I. Unusually active palladium-based catalysts for the electrooxidation of formic acid. J. Power Source 2006, 157, 78–84. [Google Scholar] [CrossRef]
- Shao, M.H.; Sasaki, K.; Adzic, R.R. Pd-Fe nanoparticles as electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2006, 128, 3526–3527. [Google Scholar] [CrossRef] [PubMed]
- Alegre, C.; Modica, E.; Lo Vecchio, C.; Siracusano, S.; Aricò, A.S.; Baglio, V. Pd supported on Ti-suboxides as bifunctional catalyst for air electrodes of metal-air batteries. Int. J. Hydrogen Energy 2016, 19579–19586. [Google Scholar] [CrossRef]
- Solis-Tobías, J.E.; Díaz-Guillén, J.A.; Meléndez-González, P.C.; Sánchez-Padilla, N.M.; Pérez-Hernández, R.; Alonso-Lemus, I.L.; Rodríguez-Varela, F.J. Enhanced catalytic activity of supported nanostructured Pd for the oxidation of organic molecules using γ-Fe2O3 and Fe3O4 as co-electrocatalysts. Int. J. Hydroggen Energy 2017, 42, 30301–30309. [Google Scholar] [CrossRef]
- Shen, T.; Zhang, J.; Chen, K.; Deng, S.; Wang, D. Recent progress of palladium-based electrocatalysts for the formic acid oxidation reaction. Energy Fuels 2020, 34, 9137–9153. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Liu, D.; Liu, H.; Qian, C.; He, H.; Yang, J. Surface composition dominates the electrocatalytic reduction of CO2 on ultrafine CuPd nanoalloys. Carbon Energy 2020, 2, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xia, Z.; Huang, Y.; Tao, L.; Chao, Y.; Yin, K.; Yang, W.; Yang, W.; Yu, Y.; Guo, S. Rh-doped PdCu ordered intermetallics for enhanced oxygen reduction electrocatalysis with superior methanol tolerance. Acta Phys. Chim. Sin. 2020, 36, 1912049. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Q.; Hou, H.; Niwa, O.; You, T. PdxCoy nanoparticle/carbon nanofiber composites with enhanced electrocatalytic properties. ACS Catal. 2014, 4, 1825–1829. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Park, D.; Jeon, S. Ultrasmall PdmMn1-mOx binary alloyed nanoparticles on graphene catalysts for ethanol oxidation in alkaline media. J. Power Source 2016, 308, 180–188. [Google Scholar] [CrossRef]
- Shinde, V.M.; Skupien, E.; Makkee, M. Synthesis of highly dispersed Pd nanoparticles supported on multi-walled carbon nanotubes and their excellent catalytic performance for oxidation of benzyl alcohol. Catal. Sci. Technol. 2015, 5, 4144–4153. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yi, Q.; Zou, T.; Zhou, X.; Nie, H. In situ deposition of Pd nanoparticles on carbon paper and their electroactivity for ethanol oxidation. Ionics 2017, 23, 3169–3176. [Google Scholar] [CrossRef]
- Lu, Y.; Du, S.; Steinberger-Wilckens, R. Three-dimensional catalyst electrodes based on PtPd nanodendrites for oxygen reduction reaction in PEFC applications. Appl. Catal. B Environ. 2016, 187, 108–114. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Kim, Y.; Hwang, Y.J.; Won, D.H. Progress in development of electrocatalyst for CO2 conversion to selective CO production. Carbon Energy 2020, 2, 72–98. [Google Scholar] [CrossRef] [Green Version]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Chen, J.; Chen, X.; Xie, Z.; Wang, X. Synthesis of “clean” and well-dispersive pd nanoparticles with excellent electrocatalytic property on graphene oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Wang, Z.L.; Green, T.C.; Henglein, A.; El-Sayed, M.A. Shape-controlled synthesis of colloidal platinum nanoparticles. Science 1996, 272, 1924–1926. [Google Scholar] [CrossRef]
- Ranjbar Bahadori, S.; Hart, R.; Hao, Y.W. Synthesis of cobalt, palladium, and rhenium nanoparticles. Tungsten 2020, 2, 261–288. [Google Scholar] [CrossRef]
- Bo Fang, L.F. PtCo-NC catalyst derived from the pyrolysis of Pt-incorporated ZIF-67 for alcohols fuel electrooxidation. Acta Phys. Chim. Sin. 2020, 36, 1905023. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, J.Y.; Han, M.; Chen, W.; Gan, L.M. Synthesis and characterization of PtRu/C catalysts from microemulsions and emulsions. J. Mater. Chem. 2002, 12, 2453–2458. [Google Scholar] [CrossRef]
- Zheng, H.; Matseke, M.S.; Munonde, T.S. The unique Pd@Pt/C core-shell nanoparticles as methanol-tolerant catalysts using sonochemical synthesis. Ultrason. Sonochem. 2019, 57, 166–171. [Google Scholar] [CrossRef]
- Kou, J.; Bennett-Stamper, C.; Varma, R.S. Green synthesis of noble nanometals (Au, Pt, Pd) using glycerol under microwave irradiation conditions. ACS Sustain. Chem. Eng. 2013, 1, 810–816. [Google Scholar] [CrossRef]
- Hou, Y.; Chang, K.; Wang, Z.; Gu, S.; Liu, Q.; Zhang, J.; Cheng, H.; Zhang, S.; Chang, Z.; Lu, Z. Rapid microwave-assisted refluxing synthesis of hierarchical mulberry-shaped Na3V2(PO4)2O2F@C as high performance cathode for sodium & lithium-ion batteries. Sci. China Mater. 2019, 62, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Fornander, H.; Birch, J.; Sandström, P.; Sundgren, J.E. Structure evolution of epitaxial Pd grown on MgO(001): A comparison between sputtering and electron-beam evaporation. Thin Solid Films 1999, 349, 4–9. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of metals and alloys from ionic liquids. J. Alloy. Compd. 2016, 654, 163–170. [Google Scholar] [CrossRef]
- Nasiri Vatan, H.; Mohammad Shafiee, M.; Khanfekr, A.; Laleh, M.; Kaffashan, H.; Jafarzadeh, K. Optimisation of experimental conditions for pulse electrodeposition of nanostructured platinum. Surf. Eng. 2014, 30, 89–96. [Google Scholar] [CrossRef]
- Lai, G.Q.; Liu, H.Z.; Chen, B.D.; Niu, D.; Lei, B.; Jiang, W.T. Electrodeposition of functionally graded Ni-W/Er2O3 rare earth nanoparticle composite film. J. Miner. Metall. Mater. 2020, 27, 818–829. [Google Scholar] [CrossRef]
- Wang, L.P.; Chen, G.; Shen, Q.X.; Li, G.M.; Guan, S.Y.; Li, B. Direct electrodeposition of ionic liquid-based template-free SnCo alloy nanowires as an anode for Li-ion batteries. J. Miner. Metall. Mater. 2018, 25, 1027–1034. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Liu, J.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Size- and density-controllable fabrication of the platinum nanoparticle/ITO electrode by pulse potential electrodeposition for ammonia oxidation. ACS Appl. Mater. Interf. 2017, 9, 27765–27772. [Google Scholar] [CrossRef]
- Stankus, D.P.; Lohse, S.E.; Hutchison, J.E.; Nason, J.A. Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ. Sci. Technol. 2011, 45, 3238–3244. [Google Scholar] [CrossRef]
- Yohannes, W.; Belenov, S.V.; Guterman, V.E.; Skibina, L.M.; Volotchaev, V.A.; Lyanguzov, N.V. Effect of ethylene glycol on electrochemical and morphological features of platinum electrodeposits from chloroplatinic acid. J. Appl. Electrochem. 2015, 45, 623–633. [Google Scholar] [CrossRef]
- Liu, J.; Hu, W.; Zhong, C.; Cheng, Y.F. Surfactant-free electrochemical synthesis of hierarchical platinum particle electrocatalysts for oxidation of ammonia. J. Power Source 2013, 223, 165–174. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Lin, Z.; Cao, Y.; Zheng, Z.; Zeng, Z.; Hu, Z. Shape-controllable pulse electrodeposition of ultrafine platinum nanodendrites for methanol catalytic combustion and the investigation of their local electric field intensification by electrostatic force microscope and finite element method. Electrochim. Acta 2014, 136, 66–74. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Liu, Y.Y.; Roy, A.K. Pulse electrodeposited Pd nanoclusters on graphene-based electrodes for proton exchange membrane fuel cells. Electrochim. Acta 2012, 64, 205–210. [Google Scholar] [CrossRef]
- Kuntyi, O.; Shepida, M.; Dobrovetska, O.; Nichkalo, S.; Korniy, S.; Eliyashevskyy, Y. Pulse electrodeposition of palladium nanoparticles onto silicon in DMSO. J. Chem. 2019, 2019, 5859204. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Ni, Z.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Improving the electrocatalytic activity of Pt monolayer catalysts for electrooxidation of methanol, ethanol and ammonia by tailoring the surface morphology of the supporting core. ChemElectroChem 2016, 3, 537–551. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Kou, Y.; Liu, Z.; Chen, X.; Li, Y.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Pt-Decorated highly porous flower-like Ni particles with high mass activity for ammonia electro-oxidation. J. Mater. Chem. A 2016, 4, 11060–11068. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Facile preparation of ultra-low Pt loading graphene-immobilized electrode for methanol oxidation reaction. Int. J. Hydrogen Energy 2018, 43, 16005–16014. [Google Scholar] [CrossRef]
- Ye, F.; Xu, C.; Liu, G.; Yuan, M.; Wang, Z.; Du, X.; Lee, J.K. Effect of pulse electrodeposition parameters on electrocatalytic the activity of methanol oxidation and morphology of Pt/C catalyst for direct methanol fuel cells. Energy Conv. Manag. 2018, 160, 85–92. [Google Scholar] [CrossRef]
- Visintin, A.; Canullo, J.C.; Triaca, W.E.; Arvia, A.J. Changes in real surface area, crystallographic orientation and morphology of platinum electrodes caused by periodic potential treatments: Phenomenological approach. J. Electroanal. Chem. 1988, 239, 67–89. [Google Scholar] [CrossRef] [Green Version]
- Perdriel, C.L.; Arvia, A.J.; Ipohorski, M. Electrochemical faceting of polycrystalline gold in 1 M H2SO4. J. Electroanal. Chem. 1986, 215, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Zhong, L.W. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Luo, W.; Hu, W.; Su, K.; Liu, F. The calculation of surface free energy based on embedded atom method for solid nickel. Appl. Surf. Sci. 2013, 265, 375–378. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, H.; Wu, P.; Zhang, F.; Wei, S.; Sun, D.; Xu, L.; Tang, Y. One-pot synthesis of freestanding porous palladium nanosheets as highly efficient electrocatalysts for formic acid oxidation. Adv. Funct. Mater. 2017, 27, 1603852. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Z.; Liu, X.; Liu, B.; Liu, J.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Tunable periodically ordered mesoporosity in palladium membranes enables exceptional enhancement of intrinsic electrocatalytic activity for formic acid oxidation. Angew. Chem. Int. Edit 2020, 59, 5092–5101. [Google Scholar] [CrossRef]
- Shao, M.; Odell, J.; Humbert, M.; Yu, T.; Xia, Y. Electrocatalysis on shape-controlled palladium nanocrystals: Oxygen reduction reaction and formic acid oxidation. J. Phys. Chem. C 2013, 117, 4172–4180. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Z.; Liu, X.; Liu, J.; Deng, Y.; Han, X.; Zhong, C.; Hu, W. Mesoporous decoration of freestanding palladium nanotube arrays boosts the electrocatalysis capabilities toward formic acid and formate oxidation. Adv. Energy Mater. 2019, 9, 1900955. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W. General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, B.; Shen, Y.; Liu, J.; Zhong, C.; Hu, W. Palladium Particles Modified by Mixed-Frequency Square-Wave Potential Treatment to Enhance Electrocatalytic Performance for Formic Acid Oxidation. Catalysts 2021, 11, 522. https://doi.org/10.3390/catal11040522
Li F, Liu B, Shen Y, Liu J, Zhong C, Hu W. Palladium Particles Modified by Mixed-Frequency Square-Wave Potential Treatment to Enhance Electrocatalytic Performance for Formic Acid Oxidation. Catalysts. 2021; 11(4):522. https://doi.org/10.3390/catal11040522
Chicago/Turabian StyleLi, Fangchao, Bin Liu, Yuanhao Shen, Jie Liu, Cheng Zhong, and Wenbin Hu. 2021. "Palladium Particles Modified by Mixed-Frequency Square-Wave Potential Treatment to Enhance Electrocatalytic Performance for Formic Acid Oxidation" Catalysts 11, no. 4: 522. https://doi.org/10.3390/catal11040522
APA StyleLi, F., Liu, B., Shen, Y., Liu, J., Zhong, C., & Hu, W. (2021). Palladium Particles Modified by Mixed-Frequency Square-Wave Potential Treatment to Enhance Electrocatalytic Performance for Formic Acid Oxidation. Catalysts, 11(4), 522. https://doi.org/10.3390/catal11040522