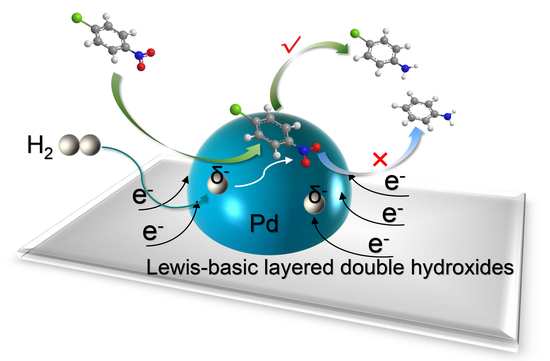
Electron-Enriched Pd Nanoparticles for Selective Hydrogenation of Halonitrobenzenes to Haloanilines
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Hydrogenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pietrowski, M. Recent developments in heterogeneous selective hydrogenation of halogenated nitroaromatic compounds to halogenated anilines. Curr. Org. Synth. 2012, 9, 470–487. [Google Scholar] [CrossRef]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of nitro compounds using 3d-non-noble metal catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gui, Q.; Yang, Z.; Tan, Z.; Guo, R.; Shi, J.-C. Synthesis of 2-substituted benzothiazoles from 1-Iodo-2-nitrobenzenes by a copper-catalyzed one-pot three-component reaction. Synthesis 2013, 45, 943–951. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhofer, G.; Pohl, M.M.; Radnik, J.; Surkus, A.E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P.; Concepcion, P.; Calvino, J.J. Transforming nonselective into chemoselective metal catalysts for the hydrogenation of substituted nitroaromatics. J. Am. Chem. Soc. 2008, 130, 8748–8753. [Google Scholar] [CrossRef]
- Zhang, J.; Pei, L.J.; Wang, J.; Zhu, P.Q.; Gu, X.M.; Zheng, Z.F. Differences in the selective reduction mechanism of 4-nitroacetophenone catalysed by rutile- and anatase-supported ruthenium catalysts. Catal. Sci. Technol. 2020, 10, 1518–1528. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.Q.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Chen, T.L.; Li, D.Q.; Jiang, H.; Xiong, C.R. High-performance Pd nanoalloy on functionalized activated carbon for the hydrogenation of nitroaromatic compounds. Chem. Eng. J. 2015, 259, 161–169. [Google Scholar] [CrossRef]
- Cardenas-Lizana, F.; Hao, Y.F.; Crespo-Quesada, M.; Yuranov, I.; Wang, X.D.; Keane, M.A.; Kiwi-Minsker, L. Selective gas phase hydrogenation of p-chloronitrobenzene over Pd catalysts: Role of the support. ACS Catal. 2013, 3, 1386–1396. [Google Scholar] [CrossRef]
- Lu, C.S.; Wang, M.J.; Feng, Z.L.; Qi, Y.N.; Feng, F.; Ma, L.; Zhang, Q.F.; Li, X.N. A phosphorus-carbon framework over activated carbon supported palladium nanoparticles for the chemoselective hydrogenation of para-chloronitrobenzene. Catal. Sci. Technol. 2017, 7, 1581–1589. [Google Scholar] [CrossRef]
- Lyu, J.H.; Wang, J.G.; Lu, C.S.; Ma, L.; Zhang, Q.F.; He, X.B.; Li, X.N. Size-dependent halogenated nitrobenzene hydrogenation selectivity of Pd nanoparticles. J. Phys. Chem. C 2014, 118, 2594–2601. [Google Scholar] [CrossRef]
- Wang, C.P.; Mao, S.J.; Wang, Z.; Chen, Y.Z.; Yuan, W.T.; Ou, Y.; Zhang, H.; Gong, Y.T.; Wang, Y.; Mei, B.B.; et al. Insight into single-atom-induced unconventional size dependence over CeO2-supported Pt catalysts. Chem 2020, 6, 752–765. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chang, C.R.; Huang, Z.Q.; Li, J.; Wu, Z.M.; Ma, Y.Y.; Zhang, Z.Y.; Wang, Y.; Qu, Y.Q. High catalytic activity and chemoselectivity of sub-nanometric Pd clusters on porous nanorods of CeO2 for hydrogenation of nitroarenes. J. Am. Chem. Soc. 2016, 138, 2629–2637. [Google Scholar] [CrossRef]
- Lu, Y.M.; Zhu, H.Z.; Li, W.G.; Hu, B.; Yu, S.H. Size-controllable palladium nanoparticles immobilized on carbon nanospheres for nitroaromatic hydrogenation. J. Mater. Chem. A 2013, 1, 3783–3788. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Shao, Y.; Wang, Y.Q.; Gates, B.C.; Xiao, F.S. A Pd@zeolite catalyst for nitroarene hydrogenation with high product selectivity by sterically controlled adsorption in the zeolite micropores. Angew. Chem. Int. Ed. 2017, 129, 9879–9883. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, Y.; Zhou, Y.; Liu, W.; Baaziz, W.; Ersen, O.; Pham-Huu, C.; Greiner, M.; Chu, W.; Wang, A.; et al. High-density and thermally stable palladium single-atom catalysts for chemoselective hydrogenations. Angew. Chem. Int. Ed. 2020, 59, 21613–21619. [Google Scholar] [CrossRef]
- Liu, H.Q.; Liang, M.H.; Xiao, C.; Zheng, N.; Feng, X.H.; Liu, Y.; Xie, J.L.; Wang, Y. An excellent Pd-based nanocomposite catalyst for the selective hydrogenation of para-chloronitrobenzene. J. Mol. Catal. A Chem. 2009, 308, 79–86. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, Y.Q.; Liu, Z.J.; Xie, C.; Feng, S.; Liu, D.D.; Shao, M.F.; Wang, S.Y. Layered double hydroxide nanosheets with multiple vacancies obtained by dry exfoliation as highly efficient oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Stepanova, L.N.; Gulyaeva, T.I.; Trenikhin, M.V.; Likholobov, V.A. Palladium supported on carbon nanoglobules as a promising catalyst for selective hydrogenation of nitroarenes. Catal. Lett. 2020, 150, 888–900. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, L.Q.; Huang, W.Q.; Xiao, Z.C.; Wu, P.F.; Zhao, W.B.; Guo, W.; Jiang, P.; Liang, M.H. Deficient copper decorated platinum nanoparticles for selective hydrogenation of chloronitrobenzene. J. Mater. Chem. A 2017, 5, 11294–11300. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Li, K.; Xiang, Y.Z.; Zhou, Y.; Wang, Q.T.; Guo, L.L.; Ma, L.; Xu, X.L.; Lu, C.S.; Feng, F.; et al. Sulfur-doped porous carbon supported palladium catalyst for high selective o-chloro-nitrobenzene hydrogenation. Appl. Catal. A Gen. 2019, 581, 74–81. [Google Scholar] [CrossRef]
- Coq, B.; Tijani, A.; Figuéras, F. Particle size effect on the kinetics of p-chloronitrobenzene hydrogenation over platinum/alumina catalysts. J. Mol. Catal. 1991, 68, 331–345. [Google Scholar] [CrossRef]
- Han, X.M.; Chen, X.; Zou, Y.; Zhang, S. Electronic state regulation of supported Pt catalysts dictates selectivity of imines/secondary amines from the cascade transformation of nitroarenes and aldehydes. Appl. Catal. B Environ. 2020, 268, 118451. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, M.X.; Wang, A.Q.; Zhang, T. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef]
- Lu, C.S.; Zhu, Q.W.; Zhang, X.J.; Liu, Q.Q.; Nie, J.J.; Fe, F.; Zhang, Q.F.; Ma, L.; Han, W.F.; Li, X.N. Preparation and catalytic performance of metal-rich Pd phosphides for solvent-free selective hydrogenation of chloronitrobenzene. Catalysts 2019, 9, 177. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.S.; Lv, J.H.; Ma, L.; Zhang, Q.F.; Feng, F.; Li, X.N. Highly selective hydrogenation of halonitroaromatics to aromatic haloamines by ligand modified Ni-based catalysts. Chin. Chem. Lett. 2012, 23, 545–548. [Google Scholar] [CrossRef]
- Frey, G.D.; Lavallo, V.; Donnadieu, B.; Schoeller, W.W.; Bertrand, G. Facile splitting of hydrogen and ammonia by nucleophilic activation at a single carbon center. Science 2007, 316, 439–441. [Google Scholar] [CrossRef] [Green Version]




| Entry | Substrates | Products | Time (h) | Conv. (%) | Sel. (%) |
| 1 |  |  | 2 | >99.9 | 91.5 |
| 2 |  |  | 4 | 98.7 | 92.8 |
| 3 |  |  | 2 | >99.9 | 99 |
| 4 |  |  | 2.5 | >99.9 | 99 |
| 5 a |  |  | 1 | >99.9 | 99 |
| 6 a |  |  | 1 | >99.9 | 99 |
| 7 a |  |  | 1 | >99.9 | 99 |
| 8 a |  |  | 1 | >99.9 | 99 |
| 9 a |  |  | 1.5 | >99.9 | 99 |
| 10 a |  |  | 3 | 89.2 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Zhang, M.; Zhang, S.; Qu, Y. Electron-Enriched Pd Nanoparticles for Selective Hydrogenation of Halonitrobenzenes to Haloanilines. Catalysts 2021, 11, 543. https://doi.org/10.3390/catal11050543
Liang Z, Zhang M, Zhang S, Qu Y. Electron-Enriched Pd Nanoparticles for Selective Hydrogenation of Halonitrobenzenes to Haloanilines. Catalysts. 2021; 11(5):543. https://doi.org/10.3390/catal11050543
Chicago/Turabian StyleLiang, Zechen, Mingkai Zhang, Sai Zhang, and Yongquan Qu. 2021. "Electron-Enriched Pd Nanoparticles for Selective Hydrogenation of Halonitrobenzenes to Haloanilines" Catalysts 11, no. 5: 543. https://doi.org/10.3390/catal11050543
APA StyleLiang, Z., Zhang, M., Zhang, S., & Qu, Y. (2021). Electron-Enriched Pd Nanoparticles for Selective Hydrogenation of Halonitrobenzenes to Haloanilines. Catalysts, 11(5), 543. https://doi.org/10.3390/catal11050543