1. Introduction
In
biology, the largest number of catalytic processes are performed by enzymes in aqueous solutions and, therefore, extremely sensitive to changes of pH in their environment. The possible pH effects on enzymes can either be inhibition or activation, acting via a variety of mechanisms and strategies, for example, by affecting the substrate binding. This has been described for invertase as early as 1911 [
1].
Additionally, for
artificial catalytic systems, it is known that the pH influences a variety of reactions: for example, in water splitting, developing well-performing catalysts for the hydrogen evolution reaction (HER) in a wide pH range is mechanistically important, because the HER elapses differently in acidic and alkaline media. Challenges therein are found to originate from electron transfer and redistribution at interfaces [
2]. Additionally, the micro- and nanostructuring of these catalysts can have a large influence on their catalytic performance and provide a handle to influence the reaction that is not available for biological systems [
3].
Most photocatalytically propelled artificial microswimmers use hydrogen peroxide (H
2O
2) as fuel. When combined with a suitable photocatalyst and a light source, H
2O
2 is decomposed in a disproportionation reaction into water and oxygen (Equation (
1)). The semiconductor thereby acts as a catalyst, which, upon illumination, provides separated electron-hole pairs to decrease the activation energy of the reaction.
The detailed course of this reaction is, to date, still a highly discussed topic [
4,
5]. It is known, that adsorption of H
2O
2 on the semiconductor surface leads to the formation of OH radicals which, in a chain reaction, form water and oxygen. Specifically in the neutral/acidic regime, a mechanism involving protons in the half reactions has been established as the most probable [
6]. However, in alkaline solutions, the dissociation of H
2O
2, which leads to the formation of HOO
−, has to be considered (
). This changes the overall reaction (Equation (
2)), also leading to an increased reaction rate [
7,
8].
In both of these scenarios, the decomposition of hydrogen peroxide will lead to the active propulsion of microswimmers, such as TiO
2 or BiVO
4. A comprehensive picture of the influence on pH and the fuel degradation mechanism is required in order to better understand the swimming behavior of microparticles in various environments, but analytical techniques for localized pH detection are still rare [
9,
10]. Despite the strong influence that might lead to better adaptation for specific tasks, most photocatalytic micromotion studies only present the behavior of the particles at neutral pH values.
In general, in order to achieve active motion, asymmetry in fuel degradation must be provided. If this is the case, propulsion is mainly caused by the two mechanisms of self-diffusiophoresis and self-electrophoresis.
In self-electrophoresis, the swimmer creates an electric field around itself, which causes the motion. On different locations of the charged swimmer surface, charged reaction products, like H
+, are created. These ions migrate around the swimmer and drag fluid with them, which, in turn, leads to a net propulsion of the swimmer [
11,
12]. In self-diffusiophoresis, a gradient is believed to be created by the localized production of a neutral solute (O
2), which drives the motion [
12]. However, it has been shown that neutral diffusiophoresis is rather unlikely when compared to an ionic mechanism, where the neutral reactants, like H
2O
2, are broken into ions first, so that this motion mechanism is also eventually dominated by self-electrophoresis [
13,
14]. It is apparent that a change in pH can dramatically influence these propulsion mechanisms. Here, we want to report our recent progress on pH modulated BiVO
4 microswimmers that propel by the photocatalytic degradation of H
2O
2. We assume their motion mechanism to be dominated by ionic contributions, which leads to a mechanism influenced by an interplay of ionic self-diffusiophoresis and electrophoresis.
For BiVO
4, Ambrosio et al. have shown that the pH of the surrounding solution will influence which species is adsorbed to the catalyst surface, which, in turn, then also influences the course of the reaction [
15,
16]. Additionally, the identity of the produced ions should have an influence on the propulsion, as hydrogen ions have a mobility twice as high as hydroxide ions [
17]. The increased reaction rate of hydrogen peroxide decomposition in alkaline media can also influence the swimmer propulsion speed. Dey et al. [
18] has also demonstrated an example for this phenomenon. Polymer microparticles were covered with Pd nanobeads and immersed in a 5% H
2O
2 solution. By imposing a pH gradient from neutral to alkaline in their solution, they observed two effects: firstly, swimmers moved at higher speeds in more alkaline solutions. Secondly, the swimmers showed pH taxis by following the gradient and navigating to the area with the highest pH. They explain this behavior by the increased self-decomposition of H
2O
2 at alkaline pH values, inducing a pressure gradient on the swimmers. Recently, we reported BiVO
4 microswimmers with adjustable motion patterns in solutions with different pH values [
19]. The stacked, square-shaped microswimmers exhibit a 90
rotation and upright motion at neutral/alkaline pH values, whereas, at acidic pH values lower than their point of zero charge (PZC) (pH
), the swimmers perform horizontal motion with absence of a rotation upon activation [
19]. To achieve more specific control over the particle motion, it is desirable to include a possibility to change the pH locally, so that the motion pattern of the same set of swimmers can be adjusted within the same solution. This way, the adjustable motion properties of these swimmers could be exploited to achieve rapid adjustment to changing conditions in their environment.
In this manuscript, we report our advances by modifying the substrates on which the microswimmers propel with a light-responsive hydroxypyrene derivative and achieve a localized influence on the solution pH by exciting the system with UV light. We investigate the influence of this functionalization on swimmer speed and motion pattern. By that, we aim to broaden the possible application range of these catalytic microswimmers into a pH range away from the neutral regime, where we want to contribute to the mechanistic understanding of H
2O
2 decomposition. Enabling pH as a handle to control microswimmer motion modes is a promising method for adjusting the swimmers propulsion to environmental conditions, as observed in many biological systems [
20].
2. Results and Discussion
Bismuth vanadate microparticles were synthesized by a solvothermal procedure, as previously reported [
19,
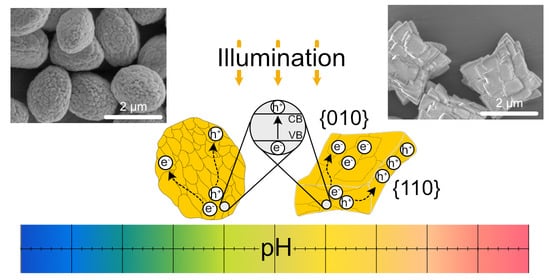
21]. By adjusting the synthesis pH precisely to 2 or 3 before particle ripening, either spheroidal or rather square shaped particles can be redeemed, respectively. For both particle shapes, SEM images reveal a polycrystalline appearance of the particles, which indicates that larger microparticles form during the synthesis by assembly of smaller crystallites (see
Figure 1a). While both of these particle shapes possess the same overall crystal structure (monoclinic scheelite), it can be seen that the square shaped particles show a kind of stacked structure, which indicates a certain orientation of the crystallites during the synthesis. This orientation of crystal facets in BiVO
4 is well known and it leads to a spatial separation of electrons and holes in an excited state [
22,
23]. As we described in a previous work, this separation leads to an asymmetric fuel degradation gradient for these particles and, consequently, to active motion when illuminated in a diluted H
2O
2 solution (see
Figure 1b) [
19].
For the spheroidal particles, a clear trend in crystal orientation cannot be easily detected. However, in combination with self-shadowing effects [
24], it may still contribute to a fuel gradient around the swimmer, as we also observe active motion for this kind of swimmers without further modification [
21].
The motion pattern of these swimmers strongly depends on the pH value of their surrounding solution.
Figure 2 illustrates this behavior for stacked square-shaped swimmers. Particle speed, motion modes, and zeta potential, together with conductivity of the solution, are displayed to give a comprehensive picture of the observed active motion. At pH values above the BiVO
4 PZC (pH = 3.4), over 95% of square shaped swimmers perform a 90
upright rotation when excited, which we call upright sliding. It is likely that the spatial crystal orientation and electron/hole separation in combination with the unique stacked square shape of these swimmers leads to the formation of osmotic pressure between the substrate and particle, causing the upright rotation upon illumination. The mean speeds are mainly constant over the different pH values, except for a strong increase around pH 10, which may be attributed to the increased reaction rate of H
2O
2 degradation in the alkaline regime. At even more alkaline conditions, the swimmer speed decreases again because of the increasing ion concentration in the solution. This may also lead to a small percentage of horizontal moving swimmers at pH 11, for which the upright rotation is suppressed. At pH values that are lower than the PZC, a rotation upon illumination does not appear anymore, which leads to horizontal sliding as a motion mode. Additionally, the mean particle speeds are slightly decreased. This observation may be explained by a closer look at the catalysts surface and the adsorbed species. When working in neutral/alkaline media above the PZC, the particles obtain a negative zeta potential, with H
2O/H
2O
2 adsorbed to the surface, which is increasingly replaced by OH
− and OOH
− in the alkaline regime. Additionally, the superoxide radical HO
2·, which is believed to be an important precursor for hydroxide radicals, is more easily adsorbed at alkaline pH values [
15,
25].
At pH values that are lower than the PZC, the surface of the particles is positively charged with hydrogen cations adsorbed to it [
15]. This can lead to the formation of stabilized, hydrogen-bonded H
2O
2 clusters, preventing an efficient fuel degradation [
25]. In consequence, lower mean particle speeds are observed. Additionally, it may be possible that this decreased catalyst activity may lead to the absence of an upright rotation upon illumination.
For spheroidal particles, trends in the change of motion pattern as well as speed are less pronounced (see
Figure S1). However, a change is also observed upon inversion of the catalysts surface charge. Here, the swimmers show no upright rotation in neutral/alkaline media and perform horizontal sliding. However, if the pH decreases below PZC, around
of the particles rotate by 90
and move upright on their tips (see
Video S1). The fact that only half of the swimmers exhibit a change in motion pattern may be attributed to the overall lower activity of these swimmers. Therefore, we decided to choose square shaped swimmers for further studies of local pH adjustment, as the effect is more pronounced for these particles and it should be possible to clearly detect the influence of a local pH change.
The influence of pH, especially on the motion pattern, can potentially be exploited to adjust the swimmer behavior to external conditions. However, adjusting the pH by adding a concentrated acid or base to a microscopic sample will always change the conditions of the whole solution and inhibit the study of flexible motion changes when a swimmer moves from one set of conditions to another one, potentially inverting its zeta potential. Here, we expect that a more localized change in pH can lead to increased control over the swimmer behavior.
To achieve this, we functionalized glass substrates with an immobilizable hydroxypyrene derivative, a so-called photoacid. These aromatic molecules are weak acids in their ground state, but, upon excitation, their acidity constant (
) increases by several orders of magnitude. This enables excited-state proton transfer (ESPT) from the photoacid to a suitable acceptor, commonly the solvent. The photoacid in this work, 3,8-bis(N,N-dimethylsulfamoyl)-6-hydroxypyrene-1-sulfonylchloride (HPDASCl), has a negative
, which makes it a super-photoacid, i.e., bearing the ability to not only transfer a proton to water, but also to non-aqueous solvents, like methanol, dimelthylformaide, or dimethyl sulfoxide [
26,
27].
To study the behavior of immobilized HPDASCl, we adapted a method that was developed by Clasen et al. to bind the dye to silica microparticles (see
Figure 3a). After functionalizing it with 3-aminopropyltriethoxysilane (APTES), it can then be bound to silica microparticles by a condensation reaction in alkaline media.
Figure 3b,c show the corresponding excitation and emission spectra of functionalized particles dispersed in deionized water. By modifying the solution pH, which was achieved by the addition of trifluoroacetic acid and sodium hydroxide, excitation and emission features of the protonized (ROH) and deprotonized (RO
−) form were obtained. For comparison, the spectra of the free HPDASCl-APTES under the same conditions can be found in
Figure S2. The deprotonized form RO
− commonly shows absorption and emission features at higher wavelengths than the protonized form ROH. In
Figure 3b, excitation spectra confirm the presence of a deprotonized form RO
− with an excitation maximum at 454 nm between pH 11 and 7. At increasingly acidic pH values, the absorption peak of the protonized form ROH at 406 nm becomes more pronounced. Emission was detected at 560 nm for all excitation spectra.
The emission spectra shown in
Figure 3c only show one emission peak at 512 nm, which can be assigned to the deprotonized form RO
−. The protonated form ROH immediately deprotonates in excited state due to the highly increased acidic constant [
27].
As the sample is excited at 365 nm, where mainly the protonated form of the dye absorbs, samples with the lowest pH typically give the highest emission intensities. Indeed, this is observed for free HPDASCl-APTES (see
Figure S2b), but the trend seems to be inversed here. The highest emission intensities are observed for rather alkaline samples, at which the dye is present in the RO
− form and no ESPT takes place. This can be an indicator that the deprotonized form of the dye is more stable when bound to the silica matrix, even in alkaline media, yielding lower ESPT rates and, therefore, lower emission intensities.
Although the pH sensitivity is less pronounced for immobilized HPDASCl as compared to the free dye (see
Figure S2), these spectra confirm its applicability in experiments with pH sensitive BiVO
4 microswimmers. The absorption and emission maxima determined here are blue-shifted up to 43 nm as compared to the values obtained by Clasen et al., who first immobilized HPDASCl on silica nanoparticles (see
Table S1) [
28]. The reason for this difference most likely lays in the larger particle size used in this work as well as the different local environment caused by solvent composition, which makes comparison with literature values more difficult.
For experiments with BiVO
4 microswimmers, the photoacid had to be immobilized on glass substrates, which was achieved by the same two-step approach, as in
Figure 3a, although the solution was in the second step dropcasted onto a clean glass substrate. The success of this method was evaluated by fluorescence microscopy of the modified substrates. The samples were excited with a 488 nm laser and fluorescence images were taken (see
Figure S3).
Figure 4a displays the mean grey value of these images versus the concentration of HPDASCl during functionalization. Concentrations of HPDASCl-APTES between
to
were chosen. For substrates with even higher concentrations during functionalization, we observed that the microswimmers just stick to the substrate upon activation, which makes the observation of their motion impossible. For the data shown, the mean grey value increases linearly with the HPDASCl-APTES concentration during functionalization over a large concentration interval of four orders of magnitude (see the blue trendline in
Figure 4a). This provides a handle to easily adjust the overall dye concentration in the final substrate to the desired amount.
The functionalization with HPDASCl should be homogeneously distributed over the whole substrate in order to reliably control the swimming mode of the microswimmers. Especially at higher concentrations, fluorescence microscopy images show that this is not the case (see
Figure S3). Instead, bright islands with high dye concentrations form. These islands also extend in the vertical axis, possibly posing obstacles to the swimmer motion.
In consequence, a third swimming mode, called tumbling, is observed when square-shaped BiVO
4 microparticles are immersed in a diluted H
2O
2 solution and excited with UV light on these substrates. When a particle tumbles, it does not clearly propel in one motion mode over the whole illumination time, but repeatedly changes between upright and horizontal.
Figure 4b shows the swimming modes observed on substrates with different dye concentration. The UV excitation of 385 nm excites the semiconductor particles as well as the photoacid at the same time. The pH drop, which is caused by ESPT of the photoacid, should then influence the swimmer motion. To assess the influence, the observations here should be compared to the data at pH 6.2 shown in
Figure 2, which is only different in terms of an unmodified substrate, but was otherwise done at the same conditions. There, all of the particles move in the upright orientation.
Undeniably, an influence on particle motion can be detected when modified substrates are used (see
Video S2). Even for samples with a dye concentration during functionalization as low as
, a non-negligible amount of horizontally swimming and tumbling microswimmers can be found, being higher than on unfunctionalized substrates at otherwise same conditions (see
Figure 2). This observation strongly suggests an influence of the increased roughness of the samples by the aforementioned formation of silane islands on the substrates. Ideally, by increasing the dye concentration during functionalization, the influence of a pH drop during excitation should tune the motion mode of the microswimmers from mainly upright to horizontal. However, in the experiments, the effect is overlaid with the increase in surface roughness stemming from the silanization reaction used for coupling. Although the functionalization clearly has an effect on the swimmer motion and increases the percentage of horizontally moving particles to up to
, this percentage does not increase linearly with increasing dye concentration.
Possibly, the inhomogeneous distribution of HPDASCl on the substrate cannot cause a constant influence on the local pH. Additionally, the before shown effect of decreased ESPT for immobilized HPDASCl may be the reason for an insufficient pH drop. This may make the particles tumble and destabilize in their upright position, but not enough to consistently overcome the point of zero charge and switch to horizontal motion.