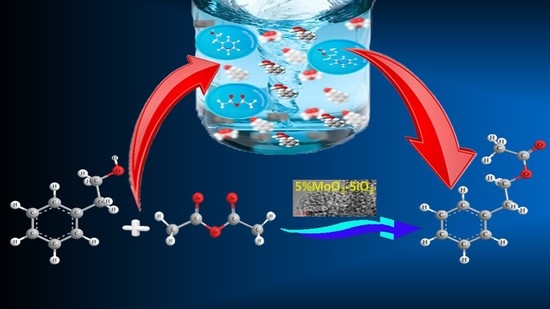
The Efficient Recyclable Molybdenum- and Tungsten-Promoted Mesoporous ZrO2 Catalysts for Aminolysis of Epoxides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Optimization of the Catalytic Variables
2.3. Catalyst Recyclability
2.4. Proposed Mechanism
3. Experiments
3.1. Materials
3.2. Catalyst Synthesis
3.3. Instrumentation
3.4. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taguchi, A.; Schuth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Wei, J.; Sun, Z.; Luo, W.; Li, Y.; Elzatahry, A.A.; Al-enizi, A.M.; Deng, Y.; Zhao, D. New insight into the synthesis of large-pore ordered mesoporous materials. J. Am. Chem. Soc. 2017, 139, 1706–1713. [Google Scholar] [CrossRef]
- Benedicte, L.; Anne, G.; Mika, L. Introduction for 20 years of research on ordered mesoporous materials. Chem. Soc. Rev. 2013, 42, 3661–3662. [Google Scholar] [CrossRef]
- Zhou, W.; Soultanidis, N.; Xu, H.; Wong, M.S.; Neurock, M.; Kiely, C.J.; Wachs, I.E. Nature of catalytically active sites in the supported WO3/ZrO2 solid acid system: A current perspective. ACS Catal. 2017, 7, 2181–2198. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, M.P.; Inagaki, S. Highly ordered mesoporous organosilica hybrid materials. Bull. Chem. Soc. Jpn. 2006, 79, 1463–1475. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.H. Solid Acids for Green Chemistry. Acc. Chem. Res. 2002, 35, 791–797. [Google Scholar] [CrossRef]
- Sayari, A. Catalysis by crystalline mesoporous molecular sieves. Chem. Mater. 1996, 8, 1840–1852. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J. Ordered mesoporous molecular sieves sythesized by a liquid-crystal templated mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Malgras, V.; Ji, Q.; Kamachi, Y.; Mori, T.; Shieh, F.-K.; Wu, K.C.-W.; Ariga, K.; Yusuke, Y. Templated synthesis for nanoarchitectured porous materials. Bull. Chem. Soc. Jpn. 2015, 88, 1171–1200. [Google Scholar] [CrossRef]
- Tanabe, K. Surface and catalytic properties of ZrO2. Mater. Chem. Phys. 1985, 13, 347–364. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, A.; Yang, J.; Su, Y.; Wang, J.; Zeng, D. Acidity of mesoporous MoOx/ZrO2 and WOx/ZrO2 materials: A combined solid-state NMR and theoretical calculation study. J. Phys. Chem. B 2006, 110, 10662–10671. [Google Scholar] [CrossRef] [PubMed]
- Bartl, M.H.; Puls, S.P.; Tang, J.; Lichtenegger, H.C.; Stucky, G.D. Cubic mesoporous frameworks with a mixed semiconductor nanocrystalline wall structure and enhanced sensitivity to visible Light. Angew. Chem. Int. Ed. 2004, 43, 3037–3040. [Google Scholar] [CrossRef]
- Williams, L.A.; Guo, N.; Motta, A.; Delferro, M.; Fragalà, I.L.; Miller, J.T.; Marks, T.J. Surface structural-chemical characterization of a single-site d0 heterogeneous arene hydrogenation catalyst having 100% active sites. Proc. Natl. Acad. Sci. USA 2013, 110, 413–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.B.G.; Lawton, M. Aluminium triflate: A remarkable Lewis acid catalyst for the ring opening of epoxides by alcohols. Org. Biomol. Chem. 2005, 3, 3269–3272. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Songstad, J. Application of the principle of hard and soft acids and bases to organic chemistry. J. Am. Chem. Soc. 1967, 39, 1827–1836. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Singh, S.; Khan, N.H.; Abdi, S.H.R.; Suresh, E.; Jasra, R.V. Efficient method for ring opening of epoxides with amines by NaY zeolite under solvent-free conditions. J. Mol. Catal. A Chem. 2007, 264, 162–169. [Google Scholar] [CrossRef]
- Sagawa, S.; Abe, H.; Hase, Y.; Inaba, T. Catalytic asymmetric aminolysis of 3,5,8-trioxabicyclo [5.1. 0] octane providing an optically pure 2-amino-1,3,4-butanetriol equivalent. J. Org. Chem. 1999, 64, 4962–4965. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Kondaskar, A.; Rudrawar, S. Scope and limitations of montmorillonite K 10 catalysed opening of epoxide rings by amines. Tetrahedron 2004, 60, 9085–9091. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Saikia, L.; Srinivas, D.; Ratnasamy, P. Regio- and stereoselective synthesis of β-amino alcohols over titanosilicate molecular sieves. Appl. Catal. A Gen. 2007, 330, 145–151. [Google Scholar] [CrossRef]
- Stöcker, M. Biofuels and biomass-to-liquid fuels in the biorefinery: Catalytic conversion of lignocellulosic biomass using porous materials. Angew. Chem. Int. Ed. 2008, 47, 9200–9211. [Google Scholar] [CrossRef]
- Falbe, J.; Bahrmann, H. Homogeneous catalysis-industrial applications. J. Chem. Educ. 1984, 61, 961–967. [Google Scholar] [CrossRef]
- Schmidt, M.; Schreiber, S.; Franz, L.; Langhoff, H.; Farhang, A.; Horstmann, M.; Drexler, H.-J.; Heller, D.; Schwarze, M. Hydrogenation of itaconic acid in micellar solutions: Catalyst recycling with cloud point extraction? Ind. Eng. Chem. Res. 2018, 58, 2445–2453. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Cullen, A. Al(OTf)3-mediated epoxide ring-opening reactions: Toward piperazine-derived physiologically active products. J. Org. Chem. 2009, 74, 9509–9512. [Google Scholar] [CrossRef]
- Krishnamurty, S.; Roy, R.K.; Vetrivel, R.; Iwata, S. The local hard-soft acid-base principle: A critical study. J. Phys. Chem. A 1997, 101, 7253–7257. [Google Scholar] [CrossRef]
- Laidler, K.J.; Glasstone, S.; Eyring, H. Application of the theory of absolute reaction rates to heterogeneous processes I. The adsorption and desorption of gases. J. Chem. Phys. 1940, 8, 659–667. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Kuo, C.-H.; Kim, E.; Meng, Y.; Seraji, M.S.; Suib, S.L. Tungsten-promoted mesoporous group 4 (Ti, Zr, and Hf) transition-metal oxides for room-temperature solvent-free acetalization and ketalization reactions. Chem. Mater. 2014, 26, 2803–2813. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Kuo, C.-H.; Biswas, S.; King’ondu, C.K.; Suib, S.L. A general approach to crystalline and monomodal pore size mesoporous materials. Nat. Commun. 2013, 4, 2952. [Google Scholar] [CrossRef] [Green Version]
- Boettcher, S.W.; Fan, J.I.E.; Tsung, C.; Shi, Q.; Stucky, G.D. Harnessing the sol-gel process for the assembly of non-silicate mesostructured oxide materials. Acc. Chem. Res. 2007, 40, 784–792. [Google Scholar] [CrossRef]
- Yamamoto, T.; Teramachi, A.; Orita, A.; Kurimoto, A.; Motoi, T.; Tanaka, T. Generation of strong acid sites on yttrium-doped tetragonal ZrO2-supported tungsten oxides: Effects of dopant amounts on acidity, crystalline phase, kinds of tungsten species, and their dispersion. J. Phys. Chem. C 2016, 120, 19705–19713. [Google Scholar] [CrossRef]
- Luan, X.; Yong, J.; Dai, X.; Zhang, X.; Qiao, H.; Yang, Y.; Zhao, H.; Peng, W.; Huang, X. Tungsten-doped molybdenum sulfide with dominant double-layer structure on mixed MgAl oxide for higher alcohol synthesis in CO hydrogenation. Ind. Eng. Chem. Res. 2018, 57, 10170–10179. [Google Scholar] [CrossRef]
- Shan, W.; Shen, W.; Li, C. Structural characteristics and redox behaviors of Ce1-xCuxOy solid solutions. Chem. Mater. 2003, 15, 4761–4767. [Google Scholar] [CrossRef]
- Vermaire, D.C.; Berge, P.C. Van The preparation of WO3/TiO2 and W03/Al2O3 and characterization reduction. J. Catal. 1989, 116, 309–317. [Google Scholar] [CrossRef]
- Iglesia, E.; Barton, D.G.; Soled, S.L.; Miseo, S.; Baumgartner, J.E.; Gates, W.E.; Fuentes, G.A.; Meitzner, G.D. Selective isomerization of alkanes on supported tungsten oxide acids. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1999; Volume 101, pp. 1–99. [Google Scholar] [CrossRef]
- Gorte, R.J. What do we know about the acidity of solid acids? Catal. Lett. 1999, 62, 1–13. [Google Scholar] [CrossRef]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef]
- Auroux, A. Determination of Acid/Base Properties by Temperature Programmed Desorption (TPD) and Adsorption Calorimetry; Springer: Dordrecht, The Netherlands, 2009; ISBN 9781402096785. [Google Scholar]
- Akinnawo, C.A.; Bingwa, N.; Meijboom, R. Metal-doped mesoporous ZrO2 catalyzed chemoselective synthesis of allylic alcohols from Meerwein–Ponndorf–Verley reduction of α,β-unsaturated aldehydes. New J. Chem. 2021, 45, 7878–7892. [Google Scholar] [CrossRef]
- Marquez, C.; Rivera-Torrente, M.; Paalanen, P.P.; Weckhuysen, B.M.; Cirujano, F.G.; De Vos, D.; De Baerdemaeker, T. Increasing the availability of active sites in Zn-Co double metal cyanides by dispersion onto a SiO2 support. J. Catal. 2017, 354, 92–99. [Google Scholar] [CrossRef]
- Kawaji, T.; Shohji, N.; Miyashita, K.; Okamoto, S. Non-Cp titanium alkoxide-based homolytic ring-opening of epoxides by an intramolecular hydrogen abstraction in b-titanoxy radical intermediates. Chem. Commun. 2011, 47, 7857–7859. [Google Scholar] [CrossRef] [Green Version]
- RajanBabu, T.V.; Nugent, W.A. Selective Generation of Free Radicals from Epoxides Using a Transition-Metal Radical. A Powerful New Tool for Organic Synthesis. J. Am. Chem. Soc. 1994, 116, 986–997. [Google Scholar] [CrossRef]
- RajanBabu, T.V.; Nugent, W.A.; Beattie, M.S. Free Radical Mediated Reduction and Deoxygenation of Epoxides. J. Am. Chem. Soc. 1990, 112, 6408–6409. [Google Scholar] [CrossRef]
- Saikia, L.; Satyarthi, J.K.; Srinivas, D.; Ratnasamy, P. Activation and reactivity of epoxides on solid acid catalysts. J. Catal. 2007, 252, 148–160. [Google Scholar] [CrossRef]
- Tang, B.; Dai, W.; Sun, X.; Wu, G.; Guan, N.; Hunger, M.; Li, L. Mesoporous Zr-Beta zeolites prepared by a post-synthetic strategy as a robust Lewis acid catalyst for the ring-opening aminolysis of epoxides. Green Chem. 2015, 17, 1744–1755. [Google Scholar] [CrossRef]







| Catalyst | Crystallite Size a (nm) | Acidity b (mmol/g) | |||
|---|---|---|---|---|---|
| 5%WO3-ZrO2 | 22 | 0.07 | 12.8 | 37.7 | 2.74 |
| 5%WO3-SiO2 | 23 | 0.02 | 3.7 | 8.1 | 1.62 |
| 5%MoO3-ZrO2 | 44 | 0.13 | 11.7 | 14.5 | 0.73 |
| 5%MoO3-SiO2 | 101 | 0.29 | 11.6 | 104.9 | 0.49 |
| Product(s) | Solvents | Selectivity (%) a | Conv. (%) | ||
|---|---|---|---|---|---|
| A | B | C b | |||
 | Cyclohexane | 100 | 0 | 88.6 | |
| Toluene | 100 | 0 | 85.3 | ||
| Hexane | 100 | 0 | 89.9 | ||
| THF | 92.6 | 0 | 7.45 | 82.1 | |
| Ethanol | 100 | 0 | 90.9 | ||
 | Cyclohexane | 94.5 | 5.47 | 14.8 | |
| Toluene | 95.3 | 4.73 | 97.6 | ||
| Hexane | 92.3 | 7.75 | 96.9 | ||
| THF | 88.6 | 0 | 11.4 | 90.2 | |
| Ethanol | 96.7 | 3.35 | 94.5 | ||
 | Cyclohexane | 91.5 | 8.55 | 97.0 | |
| Toluene | 91.6 | 8.40 | 97.4 | ||
| Hexane | 91.8 | 8.20 | 75.6 | ||
| THF | 81.1 | 6.67 | 12.3 | 90.7 | |
| Ethanol | 97.9 | 2.13 | 95.5 | ||
 | Cyclohexane | 92.6 | 7.44 | 58.9 | |
| Toluene | 91.8 | 8.24 | 91.6 | ||
| Hexane | 92.0 | 8.0 | 96.4 | ||
| THF | 85.3 | 5.31 | 9.38 | 61.5 | |
| Ethanol | 98.9 | 1.09 | 85.5 | ||
| Catalyst | Solvent | Time (min) | Catalyst Amount (mg) | Conversion (%) | Ref. |
|---|---|---|---|---|---|
| 5%MoO3-ZrO2 | Toluene | 180 | 25 | 97.4 | TW |
| Ti-MCM-41 | Toluene | 240 | 50 | 80.5 | [19] |
| TiO2 | nil | 240 | 50 | 48.0 | [19] |
| SBA-15-pr-SO3H | Solvent-free | 240 | 25 | 38.1 | [42] |
| Zr-Beta | Solvent-free | 30 | 25 | 40.5 | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlatshwayo, X.S.; Xaba, M.S.; Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. The Efficient Recyclable Molybdenum- and Tungsten-Promoted Mesoporous ZrO2 Catalysts for Aminolysis of Epoxides. Catalysts 2021, 11, 673. https://doi.org/10.3390/catal11060673
Hlatshwayo XS, Xaba MS, Ndolomingo MJ, Bingwa N, Meijboom R. The Efficient Recyclable Molybdenum- and Tungsten-Promoted Mesoporous ZrO2 Catalysts for Aminolysis of Epoxides. Catalysts. 2021; 11(6):673. https://doi.org/10.3390/catal11060673
Chicago/Turabian StyleHlatshwayo, Xolani Sibusiso, Morena S. Xaba, Matumuene Joe Ndolomingo, Ndzondelelo Bingwa, and Reinout Meijboom. 2021. "The Efficient Recyclable Molybdenum- and Tungsten-Promoted Mesoporous ZrO2 Catalysts for Aminolysis of Epoxides" Catalysts 11, no. 6: 673. https://doi.org/10.3390/catal11060673
APA StyleHlatshwayo, X. S., Xaba, M. S., Ndolomingo, M. J., Bingwa, N., & Meijboom, R. (2021). The Efficient Recyclable Molybdenum- and Tungsten-Promoted Mesoporous ZrO2 Catalysts for Aminolysis of Epoxides. Catalysts, 11(6), 673. https://doi.org/10.3390/catal11060673