A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes
Abstract
:1. Introduction
2. Methodology
3. Characteristics of Petroleum Refinery Wastewater
4. Environmental Impact of Petroleum Refinery Wastewater
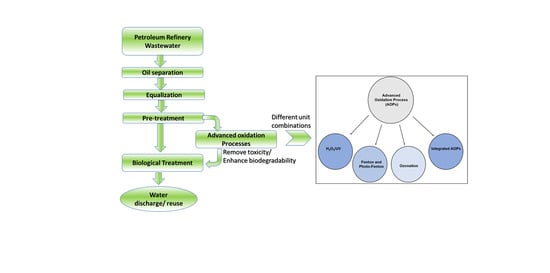
5. Advanced Oxidation Process (AOPs) for Petroleum Refinery Wastewater
5.1. Fundamentals/Chemistry of the Advanced Oxidation Process

5.2. H2O2/UV Advanced Oxidation Process and Their Application in Petroleum Refinery Wastewater
5.3. Fenton and Photo-Fenton Advanced Oxidation Processes and Their Application in Petroleum Refinery Wastewater
5.4. Ozone Based AOPs and Their Application in Petroleum Wastewater Treatment
6. Integrated AOP Processes
7. Knowledge Gaps and Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| AOP | Advanced oxidation process |
| BTEX | Benzene, Toluene, Ethylbenzene Xylene |
| RFCC | Residual Fluid Catalytic Cracking |
| UV | Ultraviolet |
| BOD | Biological oxygen demand |
| COD | Chemical oxygen demand |
| PAHs | Polycyclic aromatic hydrocarbons |
| SBBR | Spouted bed bioreactor |
| PACT | Packed activated carbon |
| EC | Electrocoagulation cell |
| ABR | Anaerobic baffled reactor |
| ASP | Activated Sludge Process |
| HF-MBR | Hollow fiber membrane bioreactors |
| PBR | Photo-bioreactors |
| EGSB-BR | Expanded granular sludge bed bioreactor |
| VOCs | Volatile Organic Compounds |
| TOC | Total organic carbon |
| O3 | Ozone |
| OH• | Hydroxyl radical |
| O2−• | Superoxide radical |
| H2O2 | Hydrogen peroxide |
| MnO2 | Manganese dioxide |
| Fe2O3 | Iron III oxide |
| TiO2 | Titanium dioxide |
| Fe2+ | Ferrous iron |
| Fe3+ | Ferric iron |
| Mn2+ | Manganese |
| Min | Minute |
| ppm | Part per million |
| Ref | Reference |
| DOC | Dissolved Organic Compounds |
| C2O42− | Oxalate ions |
| MTBE | Methyl tert-butyl ether |
| mg/L | milligram/litter |
| g/l | gram/litter |
| TPH | Total petroleum hydrocarbon |
| Ppb | Parts per billion |
| E0 | Oxidation potential |
| CHR | Chrysene |
| BbF | Benzo[b]fluoranthene |
| USEPA | United States Environmental Protection Agency |
| VFA | Volatile fatty acids |
| TDS | Total dissolved solids |
| TSS | Total suspended solids |
| DO | Dissolved oxygen |
| HEM | Hexane Extractable Material |
| MBAS | Methylene Blue Active Substance |
| API | American Petroleum Institute |
| O & G | oil and grease |
| hr | hour |
References
- Chen, Y.-C. Evaluating greenhouse gas emissions and energy recovery from municipal and industrial solid waste using waste-to-energy technology. J. Clean. Prod. 2018, 192, 262–269. [Google Scholar] [CrossRef]
- Zhang, X.; He, W.; Ren, L.; Stager, J.; Evans, P.J.; Logan, B.E. COD removal characteristics in air-cathode microbial fuel cells. Bioresour. Technol. 2015, 176, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Kumar, G.; Rene, E.R. Developments in biochar application for pesticide remediation: Current knowledge and future research directions. J. Environ. Manag. 2019, 232, 505–513. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Jiang, S.C. Current technologies and future directions for treating petroleum refineries and petrochemical plants (PRPP) wastewaters. J. Environ. Chem. Eng. 2019, 7, 103326. [Google Scholar] [CrossRef]
- Abdulredha, M.M.; Aslina, H.S.; Luqman, C.A. Overview on petroleum emulsions, formation, influence and demulsification treatment techniques. Arab. J. Chem. 2020, 13, 3403–3428. [Google Scholar] [CrossRef]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef]
- Almomani, F.A.; Shawaqfah, M.; Bhosale, R.R.; Kumar, A. Removal of emerging pharmaceuticals from wastewater by ozone-based advanced oxidation processes. Environ. Prog. Sustain. Energy 2016, 35, 982–995. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Kumar, A.; Khraisheh, M. Potential use of solar photocatalytic oxidation in removing emerging pharmaceuticals from wastewater: A pilot plant study. Solar Energy 2018, 172, 128–140. [Google Scholar] [CrossRef]
- Al Mayyahi, A.; Al-Asadi, H.A.A. Advanced oxidation processes (AOPs) for wastewater treatment and reuse: A brief review. Asian J. Appl. Sci. Technol. 2018, 2, 18–30. [Google Scholar]
- Kilic, M.Y.; Abdelraheem, W.H.; He, X.; Kestioglu, K.; Dionysiou, D.D. Photochemical treatment of tyrosol, a model phenolic compound present in olive mill wastewater, by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs). J. Hazard. Mater. 2019, 367, 734–742. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Alvarado, J.; Li, H.; Zhu, X.; Tian, Y.; Liang, L. The Advanced Oxidation Processes of Oilfield Wastewater: A Review. In Proceedings of the 4th International Conference on Water Resource and Environment (WRE 2018), Kaohsiung City, Taiwan, 17–21 July 2018. [Google Scholar]
- Ikehata, K.; Li, Y. Ozone-based processes. In Advanced Oxidation Processes for Waste Water Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–134. [Google Scholar]
- Silva, P.C.; Ferraz, N.P.; Perpetuo, E.A.; Asencios, Y.J.O. Oil produced water treatment using advanced oxidative processes: Heterogeneous-photocatalysis and photo-Fenton. J. Sediment. Environ. 2019, 4, 99–107. [Google Scholar] [CrossRef]
- Gaurav, G.K.; Yadav, D. Probing the excellence of wastewater PAHs removal approaches: A critical review. J. Contam. Hydrol. 2020, 236, 103715. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of heterogeneous catalytic ozonation for refractory organics in wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Ani, I.; Akpan, U.; Olutoye, M.; Hameed, B. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2-and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Azizah, A.N.; Widiasa, I.N. Advanced Oxidation Processes (AOPs) for Refinery Wastewater Treatment Contains High Phenol Concentration; MATEC Web of Conferences, EDP Sciences: Les Ulis, France, 2018; p. 03012. [Google Scholar]
- Lofrano, G.; Pedrazzani, R.; Libralato, G.; Carotenuto, M. Advanced oxidation processes for antibiotics removal: A review. Curr. Org. Chem. 2017, 21, 1054–1067. [Google Scholar] [CrossRef]
- de Oliveira, C.P.M.; Viana, M.M.; Amaral, M.C.S. Coupling photocatalytic degradation using a green TiO2 catalyst to membrane bioreactor for petroleum refinery wastewater reclamation. J. Water Process. Eng. 2020, 34, 101093. [Google Scholar] [CrossRef]
- Al Momani, F.A.; Shawaqfeh, A.T.; Mo‘ayyed, S.S. Solar wastewater treatment plant for aqueous solution of pesticide. Solar Energy 2007, 81, 1213–1218. [Google Scholar] [CrossRef]
- Al Momani, F.; Gonzalez, O.; Sans, C.; Esplugas, S. Combining photo-Fenton process with biological sequencing batch reactor for 2, 4-dichlorophenol degradation. Water Sci. Technol. 2004, 49, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, L.; Rahdar, A.; Bazrafshan, E.; Dahmardeh, H.; Susan, M.; Hasan, A.B.; Kyzas, G.Z. Petroleum Hydrocarbon Removal from Wastewaters: A Review. Processes 2020, 8, 447. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. A comparative study of conventional activated sludge and fixed bed hybrid biological reactor for oilfield produced water treatment: Influence of hydraulic retention time. Chem. Eng. J. 2020, 420, 127611. [Google Scholar] [CrossRef]
- Ye, H.; Liu, B.; Wang, Q.; How, Z.T.; Zhan, Y.; Chelme-Ayala, P.; Guo, S.; El-Din, M.G.; Chen, C. Comprehensive chemical analysis and characterization of heavy oil electric desalting wastewaters in petroleum refineries. Sci. Total Environ. 2020, 724, 138117. [Google Scholar] [CrossRef]
- Jain, M.; Majumder, A.; Ghosal, P.S.; Gupta, A.K. A review on treatment of petroleum refinery and petrochemical plant wastewater: A special emphasis on constructed wetlands. J. Environ. Manag. 2020, 272, 111057. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12, 770. [Google Scholar] [CrossRef] [Green Version]
- Olajire, A.A. Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-Mechanistic aspects and process chemistry perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Chen, J.; Huang, Y.; Lu, H.; Yuan, W.; Yang, Q.; Hu, J.; Yu, B.; Wang, D. Physical pretreatment of petroleum refinery wastewater instead of chemicals addition for collaborative removal of oil and suspended solids. J. Clean. Prod. 2021, 278, 123821. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2020, 403, 123970. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Lecompte, C.F.; Kakar, D.; Mehrvar, M. Photochemical treatment of benzene, toluene, ethylbenzene, and xylenes (BTEX) in aqueous solutions using advanced oxidation processes: Towards a cleaner production in the petroleum refining and petrochemical industries. J. Clean. Prod. 2018, 186, 609–617. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. An Overview of Total Petroleum Hydrocarbons. In Total Petroleum Hydrocarbons; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–27. [Google Scholar]
- Thakur, C.; Srivastava, V.C.; Mall, I.D.; Hiwarkar, A.D. Mechanistic Study and Multi-Response Optimization of the Electrochemical Treatment of Petroleum Refinery Wastewater. CLEAN–Soil Air Water 2018, 46, 1700624. [Google Scholar] [CrossRef]
- Al-Hawash, A.B.; Dragh, M.A.; Li, S.; Alhujaily, A.; Abbood, H.A.; Zhang, X.; Ma, F. Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt. J. Aquat. Res. 2018, 44, 71–76. [Google Scholar] [CrossRef]
- Putatunda, S.; Bhattacharya, S.; Sen, D.; Bhattacharjee, C. A review on the application of different treatment processes for emulsified oily wastewater. Int. J. Environ. Sci. Technol. 2019, 16, 2525–2536. [Google Scholar] [CrossRef]
- Varjani, S.J.; Joshi, R.R.; Kumar, P.S.; Srivastava, V.K.; Kumar, V.; Banerjee, C.; Kumar, R.P. Polycyclic aromatic hydrocarbons from petroleum oil industry activities: Effect on human health and their biodegradation. In Waste Bioremediation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 185–199. [Google Scholar]
- Varjani, S.J.; Gnansounou, E.; Pandey, A. Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere 2017, 188, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of industries in water scarcity and its adverse effects on environment and human health. In Environmental Concerns and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–256. [Google Scholar]
- Schweitzer, L.; Noblet, J. Water contamination and pollution. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–290. [Google Scholar]
- Armstrong, T.; Khursigara, A.J.; Killen, S.S.; Fearnley, H.; Parsons, K.J.; Esbaugh, A.J. Oil exposure alters social group cohesion in fish. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, C.; Song, M.; Dai, Q.; Jiang, L.; Zhang, D.; Zhang, G. Biodegradation of phenanthrene in polycyclic aromatic hydrocarbon-contaminated wastewater revealed by coupling cultivation-dependent and-independent approaches. Environ. Sci. Technol. 2017, 51, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Osman, A.M.; Sulieman, A. An Overview and Evaluation of Highly Porous Adsorbent Materials for Polycyclic Aromatic Hydrocarbons and Phenols Removal from Wastewater. Water 2020, 12, 2921. [Google Scholar] [CrossRef]
- Mustapha, H.I.; van Bruggen, H.J.J.A.; Lens, P.N. Vertical subsurface flow constructed wetlands for the removal of petroleum contaminants from secondary refinery effluent at the Kaduna refining plant (Kaduna, Nigeria). Environ. Sci. Pollut. Res. 2018, 25, 30451–30462. [Google Scholar] [CrossRef]
- Saleem, H.; Rehman, K.; Arslan, M.; Afzal, M. Enhanced degradation of phenol in floating treatment wetlands by plant-bacterial synergism. Int. J. Phytoremediation 2018, 20, 692–698. [Google Scholar] [CrossRef]
- Kottuparambil, S.; Kim, Y.-J.; Choi, H.; Kim, M.-S.; Park, A.; Park, J.; Shin, W.; Han, T. A rapid phenol toxicity test based on photosynthesis and movement of the freshwater flagellate, Euglena agilis Carter. Aquat. Toxicol. 2014, 155, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F. Polycyclic aromatic hydrocarbons in the Yellow River estuary: Levels, sources and toxic potency assessment. Mar. Pollut. Bull. 2017, 116, 479–487. [Google Scholar] [CrossRef]
- Badibostan, H.; Feizy, J.; Daraei, B.; Shoeibi, S.; Rajabnejad, S.H.; Asili, J.; Taghizadeh, S.F.; Giesy, J.P.; Karimi, G. Polycyclic aromatic hydrocarbons in infant formulae, follow-on formulae, and baby foods in Iran: An assessment of risk. Food Chem. Toxicol. 2019, 131, 110640. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Abu-Dieyeh, M.H.; Al Naemi, F.A.; Ahmed, T.; Al-Ghouti, M.A. environmental impact of utilization of “produced water” from oil and gas operations in turfgrass systems. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Njoku, L.; Okporuanefe, F.; Ude, E. Responses of Accessions of Zea Mays to Crude Oil Pollution Using Growth Indices and Enzyme Activities As Markers; University of Lagos: Lagos, Nigeria, 2018. [Google Scholar]
- Sathasivam, M.; Shanmugapriya, S.; Yogeshwaran, V.; Priya, A. Industrial waste water treatment using advanced oxidation process–A review. Int. J. Eng. Adv. Technol. 2019, 8, 485–488. [Google Scholar]
- Cao, J.; Yang, J.; Yue, K.; Wang, Z. Preparation of modified citrus pectin (MCP) using an advanced oxidation process with hydroxyl radicals generated by UV-H2O2. Food Hydrocoll. 2020, 102, 105587. [Google Scholar] [CrossRef]
- Li, D.; Sun, T.; Wang, L.; Wang, N. Enhanced electro-catalytic generation of hydrogen peroxide and hydroxyl radical for degradation of phenol wastewater using MnO2/Nano-G|Foam-Ni/Pd composite cathode. Electrochim. Acta 2018, 282, 416–426. [Google Scholar] [CrossRef]
- Yang, Y.; Hoffmann, M.R. Synthesis and stabilization of blue-black TiO2 nanotube arrays for electrochemical oxidant generation and wastewater treatment. Environ. Sci. Technol. 2016, 50, 11888–11894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostam, A.B.; Taghizadeh, M. Advanced oxidation processes integrated by membrane reactors and bioreactors for various wastewater treatments: A critical review. J. Environ. Chem. Eng. 2020, 8, 104566. [Google Scholar] [CrossRef]
- Qu, R.; Li, C.; Liu, J.; Xiao, R.; Pan, X.; Zeng, X.; Wang, Z.; Wu, J. Hydroxyl radical based photocatalytic degradation of halogenated organic contaminants and paraffin on silica gel. Environ. Sci. Technol. 2018, 52, 7220–7229. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Khatebasreh, M.; Mahdavianpour, M.; Lin, K.-Y.A. Oxidative removal of benzotriazole using peroxymonosulfate/ozone/ultrasound: Synergy, optimization, degradation intermediates and utilizing for real wastewater. Chemosphere 2020, 244, 125326. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar]
- Abdelraheem, W.H.; Nadagouda, M.N.; Dionysiou, D.D. Solar light-assisted remediation of domestic wastewater by NB-TiO2 nanoparticles for potable reuse. Appl. Catal. B Environ. 2020, 269, 118807. [Google Scholar] [CrossRef]
- Mishra, N.S.; Reddy, R.; Kuila, A.; Rani, A.; Mukherjee, P.; Nawaz, A.; Pichiah, S. A review on advanced oxidation processes for effective water treatment. Curr. World Environ. 2017, 12, 470. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A critical review of advanced oxidation processes for emerging trace organic contaminant degradation: Mechanisms, factors, degradation products, and effluent toxicity. J. Water Process Eng. 2020, 40, 101778. [Google Scholar] [CrossRef]
- Flouret, A.; de Almeida, M.C.; de Oliveira, T.F.; de Sá, F.P. Advanced treatment of phenol by H2O2/UV/activated carbon coupling: Influence of homogeneous and heterogeneous phase. Can. J. Chem. Eng. 2018, 96, 1979–1985. [Google Scholar] [CrossRef]
- da Silva, J.R.P.; Monteiro, M.A.; de Mendonça Ochs, S.; da Silva Moura, C.; da Fonseca, F.V.; Borges, C.P. Study of effects of pharmaceuticals on the activated sludge process combining advanced oxidation using ultraviolet/hydrogen peroxide to increase their removal and mineralization of wastewater. J. Environ. Chem. Eng. 2020, 9, 104576. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Belaqziz, M.; Wichern, M.; Lübken, M. Phytotoxicity assessment of olive mill wastewater treated by different technologies: Effect on seed germination of maize and tomato. Environ. Sci. Pollut. Res. 2020, 27, 8034–8045. [Google Scholar] [CrossRef]
- Ferreira, L.; Salmerón, I.; Peres, J.; Tavares, P.; Lucas, M.; Malato, S. Advanced Oxidation Processes as sustainable technologies for the reduction of elderberry agro-industrial water impact. Water Resour. Ind. 2020, 24, 100137. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Levchuk, I.; Manzano, M.; Sillanpää, M. Toxicity Reduction of Industrial and Municipal Wastewater by Advanced Oxidation Processes (Photo-Fenton, UVC/H2O2, Electro-Fenton and Galvanic Fenton): A Review. Catalysts 2020, 10, 612. [Google Scholar] [CrossRef]
- Rott, E.; Kuch, B.; Lange, C.; Richter, P.; Kugele, A.; Minke, R. Removal of emerging contaminants and estrogenic activity from wastewater treatment plant effluent with UV/chlorine and UV/H2O2 advanced oxidation treatment at pilot scale. Int. J. Environ. research Public Health 2018, 15, 935. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2020, 404, 124191. [Google Scholar] [CrossRef]
- Hassanshahi, N.; Karimi-Jashni, A. Comparison of photo-Fenton, O3/H2O2/UV and photocatalytic processes for the treatment of gray water. Ecotoxicol. Environ. Saf. 2018, 161, 683–690. [Google Scholar] [CrossRef]
- García, C.A.; Hodaifa, G. Real olive oil mill wastewater treatment by photo-Fenton system using artificial ultraviolet light lamps. J. Clean. Prod. 2017, 162, 743–753. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Suwan, P.; Koottatep, T.; Beck, S.E. Application of a novel, continuous-feeding ultraviolet light emitting diode (UV-LED) system to disinfect domestic wastewater for discharge or agricultural reuse. Water Res. 2019, 153, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Dhivakar, D.T. BTEX Compounds Removal from Waste Water by using UV&UV/H2O2 Process. Int. J. Recent Eng. Sci. 2018, 5, 22–25. [Google Scholar]
- Aljuboury, D.; Palaniandy, P.; Abdul Aziz, H.; Feroz, S. Treatment of petroleum wastewater by conventional and new technologies-A review. Glob. Nest J. 2017, 19, 439–452. [Google Scholar]
- GilPavas, E.; Dobrosz-Gómez, I.; Gómez-García, M.-Á. Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci. Total Environ. 2019, 651, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Neisi, A.; Afshin, S.; Rashtbari, Y.; Babaei, A.A.; Khaniabadi, Y.O.; Asadi, A.; Shirmardi, M.; Vosoughi, M. Efficiency of sequencing batch reactor for removal of organic matter in the effluent of petroleum wastewater. Data Brief 2018, 19, 2041–2046. [Google Scholar] [CrossRef]
- Raji, M.; Mirbagheri, S.A. A global trend of Fenton-based AOPs focused on wastewater treatment: A bibliometric and visualization analysis. Water Pract. Technol. 2020, 16, 19–34. [Google Scholar] [CrossRef]
- Ghime, D.; Ghosh, P. Removal of organic compounds found in the wastewater through electrochemical advanced oxidation processes: A review. Russ. J. Electrochem. 2019, 55, 591–620. [Google Scholar] [CrossRef]
- Ghasemi, H.; Aghabarari, B.; Alizadeh, M.; Khanlarkhani, A.; Abu-Zahra, N. High efficiency decolorization of wastewater by Fenton catalyst: Magnetic iron-copper hybrid oxides. J. Water Process. Eng. 2020, 37, 101540. [Google Scholar] [CrossRef]
- Zouanti, M.; Bezzina, M.; Dhib, R. Experimental study of degradation and biodegradability of oxytetracycline antibiotic in aqueous solution using Fenton process. Environ. Eng. Res. 2020, 25, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Litter, M.I.; Slodowicz, M. An overview on heterogeneous Fenton and photoFenton reactions using zerovalent iron materials. J. Adv. Oxid. Technol. 2017, 20. [Google Scholar] [CrossRef]
- Ushie, E. Simultaneous Removal of Alkylphenols and Oils in Simulated Produced Water by UV/MW/Fenton-Like Process Using a Novel Surface Functionalised Heterogeneous Pan Catalyst; De Montfort University: Leicester, UK, 2018. [Google Scholar]
- Kim, D.-H.; Lee, D.; Monllor-Satoca, D.; Kim, K.; Lee, W.; Choi, W. Homogeneous photocatalytic Fe3+/Fe2+ redox cycle for simultaneous Cr (VI) reduction and organic pollutant oxidation: Roles of hydroxyl radical and degradation intermediates. J. Hazard. Mater. 2019, 372, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhang, C.; Tang, S.; Li, X.; Tang, J.; Rao, Y.; Wang, Z.; Zhang, Q. Enhancing CaO2 fenton-like process by Fe (II)-oxalic acid complexation for organic wastewater treatment. Water Res. 2019, 163, 114861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, X.; Shao, Z.; Shan, D.; Li, G.; Irini, A. Comparison of Fenton, UV-Fenton and nano-Fe3O4 catalyzed UV-Fenton in degradation of phloroglucinol under neutral and alkaline conditions: Role of complexation of Fe3+ with hydroxyl group in phloroglucinol. Chem. Eng. J. 2017, 313, 938–945. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, L.; Lei, J.; Liu, Y.; Zhang, J. Photo-Fenton degradation of phenol by CdS/rGO/Fe2+ at natural pH with in situ-generated H2O2. Appl. Catal. B Environ. 2019, 241, 367–374. [Google Scholar] [CrossRef]
- Thakare, Y.D.; Wani, K.S. Treatment of Industrial Waste Water by Fenton Process. J. Archit. Technol. 2019, XI, 43–49. [Google Scholar]
- Villegas-Guzman, P.; Giannakis, S.; Rtimi, S.; Grandjean, D.; Bensimon, M.; De Alencastro, L.F.; Torres-Palma, R.; Pulgarin, C. A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl. Catal. B Environ. 2017, 219, 538–549. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Zhang, Y.; Shen, F.; Yang, G.; Zhang, X.; Wang, L.; Luo, L.; He, Y.; Deng, S. Degradation process and kinetics study of actual urotropine wastewater by Fenton method. Desalination Water Treat. 2019, 160, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Perini, J.A.L.; Tonetti, A.L.; Vidal, C.; Montagner, C.C.; Nogueira, R.F.P. Simultaneous degradation of ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine, and disinfection of hospital effluent after biological treatment via photo-Fenton process under ultraviolet germicidal irradiation. Appl. Catal. B Environ. 2018, 224, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Shokri, A. Application of Sono–photo-Fenton process for degradation of phenol derivatives in petrochemical wastewater using full factorial design of experiment. Int. J. Ind. Chem. 2018, 9, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Danforth, R.A. Ultrafast Photochemistry of Aqueous Iron (III) Complexes. Ph.D. Thesis, College of Letters & Science, Montana State University-Bozeman, Bozeman, MT, USA, 2017. [Google Scholar]
- Muramatsu, K.; Tokumura, M.; Wang, Q.; Miyake, Y.; Amagai, T.; Makino, M. Mitigation of the inhibitory effects of co-existing substances on the Fenton process by UV light irradiation. J. Environ. Sci. Health Part A 2020, 55, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-x.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Sirés, I.; Zhang, H.; Huang, Y.-H. Mineralization of pentachlorophenol by ferrioxalate-assisted solar photo-Fenton process at mild pH. Chemosphere 2019, 217, 475–482. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process Saf. Environ. Prot. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S.; Moussavi, M. Advances in Fenton and Fenton based oxidation processes for industrial effluent contaminants control-a review. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–18. [Google Scholar]
- Fernandes, A.; Makoś, P.; Boczkaj, G. Treatment of bitumen post oxidative effluents by sulfate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean. Prod. 2018, 195, 374–384. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef] [PubMed]
- Dalari, B.L.S.K.; Giroletti, C.L.; Dalri-Cecato, L.; Domingos, D.G.; Hassemer, M.E.N. Application of heterogeneous photo-fenton process using chitosan beads for textile wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103893. [Google Scholar] [CrossRef]
- Lumbaque, E.C. Degradation of Pharmaceuticals in Hospital Wastewater by Solar Photo-Fenton Processes; UFRGS: Porto Alegre, Brazil, 2020. [Google Scholar]
- Nikravesh, B.; Shomalnasab, A.; Nayyer, A.; Aghababaei, N.; Zarebi, R.; Ghanbari, F. UV/Chlorine process for dye degradation in aqueous solution: Mechanism, affecting factors and toxicity evaluation for textile wastewater. J. Environ. Chem. Eng. 2020, 8, 104244. [Google Scholar] [CrossRef]
- Amildon, I.; Paniagua, C.E.; Paiva, V.A.; Gonçalves, B.R.; Sousa, R.M.; Machado, A.E.; Trovó, A.G. Degradation and initial mechanism pathway of chloramphenicol by photo-Fenton process at circumneutral pH. Chem. Eng. J. 2018, 339, 531–538. [Google Scholar] [CrossRef]
- Pourehie, O.; Saien, J. Homogeneous solar Fenton and alternative processes in a pilot-scale rotatable reactor for the treatment of petroleum refinery wastewater. Process Saf. Environ. Prot. 2020, 135, 236–243. [Google Scholar] [CrossRef]
- El-Qanni, A. Development of Sustainable Nanosorbcats Based Technology for Hydrocarbons and Organic Pollutants Recovery from Industrial Wastewater; University of Calgary: Calgary, Alberta, 2017. [Google Scholar]
- Radwan, M.; Alalm, M.G.; El-Etriby, H.K. Application of electro-Fenton process for treatment of water contaminated with benzene, toluene, and p-xylene (BTX) using affordable electrodes. J. Water Process Eng. 2019, 31, 100837. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Hassan, A.K.; Hassan, M.M.A.; Hasan, A.F. Treatment of Iraqi Petroleum Refinery Wastewater by Advanced Oxidation Processes; Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; p. 012071. [Google Scholar]
- Pintor, A.M.; Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Testolin, R.C.; Mater, L.; Sanches-Simões, E.; Dal Conti-Lampert, A.; Corrêa, A.X.; Groth, M.L.; Oliveira-Carneiro, M.; Radetski, C.M. Comparison of the mineralization and biodegradation efficiency of the Fenton reaction and Ozone in the treatment of crude petroleum-contaminated water. J. Environ. Chem. Eng. 2020, 8, 104265. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A.; Makoś, P. Study of different advanced oxidation processes for wastewater treatment from petroleum bitumen production at basic pH. Ind. Eng. Chem. Res. 2017, 56, 8806–8814. [Google Scholar] [CrossRef]
- Cruz Alcalde, A. Contribution to Performance Characterization and Kinetic Modelling of Micropollutants Abatement in Water and Wastewater by Ozone-based Oxidation Processes. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- Contreras, S.; Rodrıguez, M.; Al Momani, F.; Sans, C.; Esplugas, S. Contribution of the ozonation pre-treatment to the biodegradation of aqueous solutions of 2, 4-dichlorophenol. Water Res. 2003, 37, 3164–3171. [Google Scholar] [CrossRef]
- Kim, M.S.; Cha, D.; Lee, K.-M.; Lee, H.-J.; Kim, T.; Lee, C. Modeling of ozone decomposition, oxidant exposures, and the abatement of micropollutants during ozonation processes. Water Res. 2020, 169, 115230. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater-A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Malvestiti, J.A.; Dantas, R.F. Disinfection of secondary effluents by O3, O3/H2O2 and UV/H2O2: Influence of carbonate, nitrate, industrial contaminants and regrowth. J. Environ. Chem. Eng. 2018, 6, 560–567. [Google Scholar] [CrossRef]
- Wen, C.; Wang, H.; Wang, L.; Lou, Z.; Sun, Z.; Zhou, Z. The reduction of waste lubricant oil distillate through the enhancement of organics degradation by ozonation with elevated temperature and stable pH for the zero discharge. J. Clean. Prod. 2019, 240, 118194. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.; Lim, J. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012089. [Google Scholar] [CrossRef]
- Shahrezaei, F.; Mansouri, Y.; Zinatizadeh, A.A.L.; Akhbari, A. Process modeling and kinetic evaluation of petroleum refinery wastewater treatment in a photocatalytic reactor using TiO2 nanoparticles. Powder Technol. 2012, 221, 203–212. [Google Scholar] [CrossRef]
- Tao, P.; Yang, C.; Wang, H.; Zhao, Y.; Zhang, X.; Shao, M.; Sun, T. Synergistic effects of ultrasonic-assisted ozonation on the formation of hydrogen peroxide. J. Environ. Chem. Eng. 2020, 9, 104905. [Google Scholar] [CrossRef]
- Liu, Z.; Chys, M.; Yang, Y.; Demeestere, K.; Van Hulle, S. Oxidation of trace organic contaminants (TrOCs) in wastewater effluent with different ozone-based AOPs: Comparison of ozone exposure and •OH formation. Ind. Eng. Chem. Res. 2019, 58, 8896–8902. [Google Scholar] [CrossRef]
- Al Momani, F. Impact of photo-oxidation technology on the aqueous solutions of nitrobenzene: Degradation efficiency and biodegradability enhancement. J. Photochem. Photobiol. A Chem. 2006, 179, 184–192. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Buehlmann, P.H. Balancing Bromate Formation, Organics Oxidation, and Pathogen Inactivation: The Impact of Bromate Suppression Techniques on Ozonation System Performance in Reuse Waters. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2019. [Google Scholar]
- El-Din, M.G.; Smith, D.W.; Al Momani, F.; Wang, W. Oxidation of resin and fatty acids by ozone: Kinetics and toxicity study. Water Res. 2006, 40, 392–400. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Hai, F.I. A critical review on advanced oxidation processes for the removal of trace organic contaminants: A voyage from individual to integrated processes. Chemosphere 2020, 260, 127460. [Google Scholar] [CrossRef]
- Al Momani, F. Degradation of cyanobacteria anatoxin-a by advanced oxidation processes. Sep. Purif. Technol. 2007, 57, 85–93. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017, 33, 49–89. [Google Scholar] [CrossRef]
- Yang, S.; Song, Y.; Chang, F.; Wang, K. Evaluation of chemistry and key reactor parameters for industrial water treatment applications of the UV/O3 process. Environ. Res. 2020, 188, 109660. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Esplugas, S.; Sans, C. Continuous versus single H2O2 addition in peroxone process: Performance improvement and modelling in wastewater effluents. J. Hazard. Mater. 2020, 387, 121993. [Google Scholar] [CrossRef]
- Al Momani, F.A.; Jarrah, N. Treatment and kinetic study of cyanobacterial toxin by ozone. J. Environ. Sci. Health Part A 2010, 45, 719–731. [Google Scholar] [CrossRef]
- Biard, P.-F.; Dang, T.T.; Bocanegra, J.; Couvert, A. Intensification of the O3/H2O2 advanced oxidation process using a continuous tubular reactor filled with static mixers: Proof of concept. Chem. Eng. J. 2018, 344, 574–582. [Google Scholar] [CrossRef]
- Meshref, M.N.; Klamerth, N.; Islam, M.S.; McPhedran, K.N.; El-Din, M.G. Understanding the similarities and differences between ozone and peroxone in the degradation of naphthenic acids: Comparative performance for potential treatment. Chemosphere 2017, 180, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhan, J.; Yao, W.; Wang, B.; Deng, S.; Huang, J.; Yu, G.; Wang, Y. Comparison of pharmaceutical abatement in various water matrices by conventional ozonation, peroxone (O3/H2O2), and an electro-peroxone process. Water Res. 2018, 130, 127–138. [Google Scholar] [CrossRef]
- Khatri, I.; Singh, S.; Garg, A. Performance of electro-Fenton process for phenol removal using Iron electrodes and activated carbon. J. Environ. Chem. Eng. 2018, 6, 7368–7376. [Google Scholar] [CrossRef]
- Ahmadi, M.; Jorfi, S.; Kujlu, R.; Ghafari, S.; Soltani, R.D.C.; Haghighifard, N.J. A novel salt-tolerant bacterial consortium for biodegradation of saline and recalcitrant petrochemical wastewater. J. Environ. Manag. 2017, 191, 198–208. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kazemi, H.; Mirbagheri, S.; Rockaway, T.D. An optimized biological approach for treatment of petroleum refinery wastewater. J. Environ. Chem. Eng. 2016, 4, 3401–3408. [Google Scholar] [CrossRef]
- Mirbagheri, S.A.; Ebrahimi, M.; Mohammadi, M. Optimization method for the treatment of Tehran petroleum refinery wastewater using activated sludge contact stabilization process. Desalination Water Treat. 2014, 52, 156–163. [Google Scholar] [CrossRef]
- Liang, J.; Mai, W.; Tang, J.; Wei, Y. Highly effective treatment of petrochemical wastewater by a super-sized industrial scale plant with expanded granular sludge bed bioreactor and aerobic activated sludge. Chem. Eng. J. 2019, 360, 15–23. [Google Scholar] [CrossRef]
- Razavi, S.M.R.; Miri, T. A real petroleum refinery wastewater treatment using hollow fiber membrane bioreactor (HF-MBR). J. Water Process Eng. 2015, 8, 136–141. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Alhaija, M.A.; Al-Zuhair, S. Evaluation of a three-step process for the treatment of petroleum refinery wastewater. J. Environ. Chem. Eng. 2014, 2, 56–62. [Google Scholar] [CrossRef]
- An, C.; Huang, G.; Yao, Y.; Zhao, S. Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci. Total Environ. 2017, 579, 537–556. [Google Scholar] [CrossRef]
- Huo, S.; Zhu, F.; Zou, B.; Xu, L.; Cui, F.; You, W. A two-stage system coupling hydrolytic acidification with algal microcosms for treatment of wastewater from the manufacture of acrylonitrile butadiene styrene (ABS) resin. Biotechnol. Lett. 2018, 40, 689–696. [Google Scholar] [CrossRef]
- Fernandes, A.; Gągol, M.; Makoś, P.; Khan, J.A.; Boczkaj, G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol. 2019, 224, 1–14. [Google Scholar] [CrossRef]
- Dastpak, H.; Pasalari, H.; Jafari, A.J.; Gholami, M.; Farzadkia, M. Improvement of Co-Composting by a combined pretreatment Ozonation/Ultrasonic process in stabilization of raw activated sludge. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Zhai, J.; Ma, H.; Liao, J.; Rahaman, M.; Yang, Z.; Chen, Z. Comparison of Fenton, ultraviolet–Fenton and ultrasonic–Fenton processes on organics and colour removal from pre-treated natural gas produced water. Int. J. Environ. Sci. Technol. 2018, 15, 2411–2422. [Google Scholar] [CrossRef]
- Shah, A.; Shah, M. Characterisation and bioremediation of wastewater: A review exploring bioremediation as a sustainable technique for pharmaceutical wastewater. Groundw. Sustain. Dev. 2020, 11, 100383. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Luster, E.; Lozzi, L.; Luxbacher, T.; Mamane, H. MS2 bacteriophage inactivation using a N-doped TiO2-coated photocatalytic membrane reactor: Influence of water-quality parameters. Chem. Eng. J. 2018, 354, 995–1006. [Google Scholar] [CrossRef]
- Yadav, D.N.; Kishore, K.A.; Bethi, B.; Sonawane, S.H.; Bhagawan, D. ZnO nanophotocatalysts coupled with ceramic membrane method for treatment of Rhodamine-B dye waste water. Environ. Dev. Sustain. 2018, 20, 2065–2078. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Dindi, A.; Balogun, H.A. Polygeneration in desalination by photovoltaic thermal systems: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 130, 109946. [Google Scholar] [CrossRef]







| Composition of Each Element | Composition in Petroleum Wastewater | Limitations | Metal Compositions | Components in Petroleum Wastewater | Limitations |
|---|---|---|---|---|---|
| pH | 4.3–10 | 6.5–8.5 | Ca | 18–132,687 | 100 |
| Density | 1014–1140 | - | Na | 316–134,652 | - |
| TOC | 3.4–5960 | - | K | 8.6–14,649 | - |
| COD | 1200 | ≤50 | Mg | 4–18,145 | 100 |
| BOD | - | ≤30 | Fe | <0.1–100 | 0.3 |
| TSS | 1.2–21,820 | 30 | Al | 310–410 | ≤0.2 |
| TDS | 1 × 103–4 × 105 | 1200 | B | 5.00–95 | 1 |
| DO | 8.2 | <4.0 | Ba | 0–22400 | 1 |
| TPH | 2–565 | - | Cd | <0.005–0.2 | 0.005 |
| BTEX | 0.39–35 | - | Cr | 0.02–1.1 | ≤0.1 |
| base and neutrals | <140 | - | Cu | <0.002–1.5 | 1.3 |
| Cl | 80–310,561 | 1400–190,000 | Li | 3–50.00 | - |
| Br | 0–12,000 | 150–1149 | Mn | <0.004–175 | 0.05 |
| I | 0–500 | - | Ni | - | 0.3 |
| HCO3 | 1.9–7355 | - | Pb | 0.002–8.8 | 0.015 |
| CO3 | 0–800 | - | Sr | 0.02–1000 | - |
| SO4 | 0.5–7851 | <0.1–47 | Ti | <0.01–0.7 | - |
| PO4 | 0–0.10 | - | Zn | 0.01–35 | 7.4 |
| SO3 | ~10 | - | As | <0.005–0.3 | 0.02 |
| NO3 | 0–3.5 | - | Hg | <0.001–0.002 | 0.005 |
| NO2 | 0.05 | - | Ag | <0.001–0.15 | 0.1 |
| NH3-N | 10–300 | - | Be | <0.001–0.004 | - |
| Total Polar | 9.7–600 | - | NORM (pCi/L) | - | - |
| Higher acids | <1–63 | - | Total Ra | 0.054–32,400 | 5 |
| Phenols | till 23 | - | U | 0.008–2.7 | - |
| VFA | 2–4900 | - | Th | 0.008–0.027 | 15 |
| Oil & Grease | 6.9–210 | 2.3–60 | Pb | 1.35–5130 | - |
| m-xylene | 0.01–54 | - | Po | 0.005–0.17 | - |
| MBAS | 0.01–54 | - | - | - | - |
| HEM | 0.6–2000 | 0.02 | - | - | - |
| Alkalinity | 6.1–200 | - | - | - | - |
| Contaminant | Environmental Impact | Ref |
|---|---|---|
| Phenol | Have harmful effects on the muscles, causing moving problems, pain to the gastrointestinal system, and even death. | Yang et al. (2020) |
| Methyl-butyl ether | In an aquatic environment, it quickly causes the reduction in dissolved oxygen. Regarding human health, it causes kidney and blood cancers and affects the nerve. | Gallo et al. (2020) |
| Benzene | The existence in the aquatic environment affects the stability and health of the organisms, leading to a law regenerative rate, and affecting the behavior of the organisms. It also reduces the growth of plants and animals, and, with long time exposure, it can lead to their death. | Rabani et al. (2020) |
| Toluene | Have harmful impacts on the different types of microorganism (aquatic, soil, etc). | Poyraz et al. (2020) |
| Ethylbenzene | Exposure for long periods affects the kidney which can lead to pain in the inner ear. | Wollin et al. (2020) |
| Xylene | Affects the kidney, nervous system, and the dysfunctions of the liver. In addition, various negative impacts on the neurological system have been stated. | Egendorf et al. (2020) |
| Non-photochemical-Homogeneous Methods | Photochemical-Homogeneous Methods | Non-Photochemical-Heterogeneous Processes | Photochemical-Heterogeneous Processes |
|---|---|---|---|
|
|
|
|
| Process | Type of Wastewater (WW) | Conditions | Efficiency | Comments and Details | Ref |
|---|---|---|---|---|---|
| TiO2/UV | Model: TPH Benzene (Toluene Phenol) Naphthalene | [TiO2] 100 mg/L pH 6.5 for 1 h | COD reduction from 970 mg/L to 65 mg/L = 93% removal at 90 min, 30 °C and pH 3 using 100 mg/L | Higher degradations rates for with TiO2/UV = 92%, 98.8%, 91.5%, and 93% for B, T, P and N, respectively. | Ihtisham et al. 2020 |
| H2O2/UV | Model [Phenol] 100 mg/L | (H2O2) 3060 mg/L pH 9 | 99% phenol removal in 60 min | H2O2/UV<O3/UV 0.081 min−1 < 0.0881 min−1 | Alrousan et al. 2020 |
| H2O2/UV | Oil COD 9000 ± 500 mg/L | (H2O2) 3330 mg/L pH 12 | 40% COD removal in 150 min | Negative impact on COD removal at basic pH | GilPavas et al. 2019 |
| H2O2/UV | Model: [nonylphenol] 4.41 mg/L | (H2O2) 1.7–17 mg/L pH 11 | To achieve 90% of degradation [H2O2] 1.7 mg/L in 20 min (H2O2) 3.4 mg/L in 16 min (H2O2) 8.5 mg/L in 12 min | At pH 7 the time needed was 3 times higher in all concentrations studied | Kaur et al. 2020 |
| H2O2 H2O2/UV | Linear Alkyl Benzene COD 300–350 mg/L | (H2O2) 340 mg/L pH 9 | H2O2: 27% COD removal in 180 min H2O2/UV: 36% COD removal in 180 min | H2O2 (27%) < O3/UV, H2O2/UV (36%) < O3, (37%) < H2O2/O3 (39%)< H2O2/O3/UV (42%) | Fernandes et al. 2019 |
| H2O2/UV | Model: MTBE 25 mg/L | (H2O2) 34 mg/L pH 12 | 98% MTBE removal in 60 min | H2O2/UV (0.126 min−1) | Neisi et al. 2018 |
| No | Method | Parameter | Removal Efficiency (%) | pH | H2O2 (ppm) | Fe2+ (ppm) | Ratio of H2O2:Fe2+ | Reaction Time (min) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | H2O2/Fe2+/solar | TOC | 84 | 2 | 1 | 0.08 | 12.5 | 300 | Aljuboury et al. 2017 |
| 2 | H2O2/Fe2+ | COD/ TOC | 56 COD 54 TOC | 4.3 | 9.7 | 1.1 | 8.8 | 120 | Edison et al. 2019 |
| 3 | H2O2/Fe2+/UV | COD | 92 | 3 | 110 | 35 | 3.14 | 92 | Majed et al. 2020 |
| 4 | H2O2/Fe2+/solar | COD | 84 | 3.2 | 200 | 1.5 | 133 | 180 | Asaithambi et al. 2017 |
| 5 | H2O2/Fe3+ | TOC | 90 | 3 | 500 | 250 | 2 | 120 | Deng et al. 2017 |
| 6 | H2O2/Fe2+/UV | COD | 72 | 5.6 | 17.86 | 1.76 | 10.14 | 70 | Shokri et al. 2019 |
| 7 | H2O2/Fe3+/TiO2 | COD | 69.6 | 3 | 1600 | 30 | 53 | 60 | Hassan 2018 |
| 8 | H2O2/Fe2+/UV | COD | 76.8 | 3 | 250 | 40 | 6.25 | 30 | Tufaner 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmobarak, W.F.; Hameed, B.H.; Almomani, F.; Abdullah, A.Z. A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts 2021, 11, 782. https://doi.org/10.3390/catal11070782
Elmobarak WF, Hameed BH, Almomani F, Abdullah AZ. A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts. 2021; 11(7):782. https://doi.org/10.3390/catal11070782
Chicago/Turabian StyleElmobarak, Wamda Faisal, Bassim H. Hameed, Fares Almomani, and Ahmad Zuhairi Abdullah. 2021. "A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes" Catalysts 11, no. 7: 782. https://doi.org/10.3390/catal11070782
APA StyleElmobarak, W. F., Hameed, B. H., Almomani, F., & Abdullah, A. Z. (2021). A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts, 11(7), 782. https://doi.org/10.3390/catal11070782