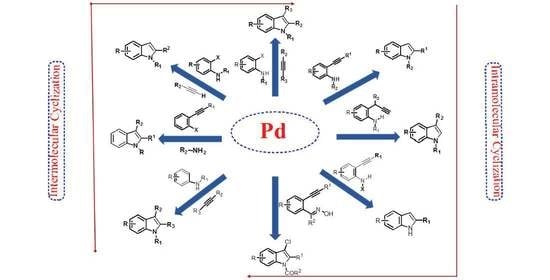
Synthesis of Indoles via Intermolecular and Intramolecular Cyclization by Using Palladium-Based Catalysts
Abstract
:1. Introduction
2. Indole Synthesis
2.1. Indole Synthesis via Ortho-Alkynyl Anilines
2.2. Indole Synthesis via Cascade Reaction
2.3. Indole Synthesis via Annulations of Non-Terminal Alkynes
2.4. Indole Synthesis via C-H Activation
2.5. Indole Synthesis via Hydroamination or N-Arylation
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Order, R.H. Lindwall. Indole. Chem. Rev. 1942, 30, 69–96. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P.; Pathak, D. Biological importance of the indole nucleus in recent years: A comprehensive review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Abele, E.; Abele, R.; Dzenitis, O.; Lukevics, E. Indole and Isatin Oximes: Synthesis, Reactions, and Biological Activity. Chem. Heterocycl. Compd. 2003, 39, 3–35. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Kruk, I.; Lichszteld, K.; Michalska, T.; Kladna, A.; Marczynski, S.; Ölgen, S. Scavenging of reactive oxygen species by N-substituted indole-2 and 3-carboxamides. J. Lumin. 2004, 19, 1–7. [Google Scholar] [CrossRef]
- Al-Hiari, Y.M.; Qaisi, A.M.; El-Abadelah, M.M.; Voelter, W. Synthesis and antibacterial activity of some substituted 3-(aryl)-and 3-(heteroaryl) indoles. Monatsh. Chem. 2006, 137, 243–248. [Google Scholar] [CrossRef]
- Barraja, P.; Sciabica, L.; Diana, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G.; Disarò, S.; Basso, G. Synthesis and photochemotherapeutic activity of thiopyrano [2, 3-e] indol-2-ones. Bioorgan. Med. Chem. Lett. 2005, 15, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.C.; Jiang, Y.F.; Chang, Y.L.; Lee, S.J. Synthesis and Cytotoxicity Studies of Cyclohepta [b] indoles, Benzo [6, 7] Cyclohepta [1, 2-b] Indoles, Indeno [1, 2-b] Indoles, and Benzo [a] Carbazoles. J Chin Chem Soc. 2006, 53, 647–662. [Google Scholar] [CrossRef]
- Kalaskar, G.; Girisha, M.; Purohit, M.; Thippeswamy, B.; Patil, B. Synthesis and evaluation of in vivo antiinflammatory activity of indole-3-acetic acids. Indian J. Heterocycl. Chem. 2007, 16, 325–328. [Google Scholar]
- Li, Y.-Y.; Wu, H.-S.; Tang, L.; Feng, C.-R.; Yu, J.-H.; Li, Y.; Yang, Y.-S.; Yang, B.; He, Q.-J. The potential insulin sensitizing and glucose lowering effects of a novel indole derivative in vitro and in vivo. Pharmacol. Res. 2007, 56, 335–343. [Google Scholar] [CrossRef]
- Radwan, M.A.; Ragab, E.A.; Sabry, N.M.; El-Shenawy, S.M. Synthesis and biological evaluation of new 3-substituted indole derivatives as potential anti-inflammatory and analgesic agents. Bioorgan. Med. Chem. 2007, 15, 3832–3841. [Google Scholar] [CrossRef]
- Talaz, O.; Gülçin, I.; Göksu, S.; Saracoglu, N. Antioxidant activity of 5, 10-dihydroindeno [1, 2-b] indoles containing substituents on dihydroindeno part. Bioorgan. Med. Chem. 2009, 17, 6583–6589. [Google Scholar] [CrossRef]
- Bellemin, R.; Decerprit, J.; Festal, D. New indole derivatives as ACAT inhibitors: Synthesis and structure-activity relationships. Eur. J. Med. Chem. 1996, 31, 123–132. [Google Scholar] [CrossRef]
- Grumel, V.; Mérour, J.-Y.; Lesur, B.; Giboulot, T.; Frydman, A.; Guillaumet, G. Design and synthesis of a series of indole glycoprotein IIb/IIIa inhibitors. Eur. J. Med. Chem. 2002, 37, 45–62. [Google Scholar] [CrossRef]
- McKew, J.C.; Foley, M.A.; Thakker, P.; Behnke, M.L.; Lovering, F.E.; Sum, F.-W.; Tam, S.; Wu, K.; Shen, M.W.; Zhang, W. Inhibition of cytosolic phospholipase A2α: Hit to lead optimization. J. Med. Chem. 2006, 49, 135–158. [Google Scholar] [CrossRef]
- Merino, I.; Monge, A.; Font, M.; de Irujo, J.J.M.; Alberdi, E.; Santiago, E.; Prieto, I.; Lasarte, J.J.; Sarobe, P.; Borrás, F. Synthesis and anti-HIV-1 activities of new pyrimido [5, 4-b] indoles. Farmaco 1999, 54, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Takami, H.; Kishibayashi, N.; Ishii, A.; Kumazawa, T. Indole and benzimidazole derivatives as steroid 5α-reductase inhibitors in the rat prostate. Bioorgan. Med. Chem. 1998, 6, 2441–2448. [Google Scholar] [CrossRef]
- Zheng, M.; Zheng, M.; Ye, D.; Deng, Y.; Qiu, S.; Luo, X.; Chen, K.; Liu, H.; Jiang, H. Indole derivatives as potent inhibitors of 5-lipoxygenase: Design, synthesis, biological evaluation, and molecular modeling. Bioorg. Med. Chem. Lett. 2007, 17, 2414–2420. [Google Scholar] [CrossRef]
- Konkel, M.J.; Lagu, B.; Boteju, L.W.; Jimenez, H.; Noble, S.; Walker, M.W.; Chandrasena, G.; Blackburn, T.P.; Nikam, S.S.; Wright, J.L. 3-arylimino-2-indolones are potent and selective galanin GAL3 receptor antagonists. J. Med. Chem. 2006, 49, 3757–3758. [Google Scholar] [CrossRef]
- Mahindroo, N.; Wang, C.-C.; Liao, C.-C.; Huang, C.-F.; Lu, I.-L.; Lien, T.-W.; Peng, Y.-H.; Huang, W.-J.; Lin, Y.-T.; Hsu, M.-C. Indol-1-yl acetic acids as peroxisome proliferator-activated receptor agonists: Design, synthesis, structural biology, and molecular docking studies. J. Med. Chem. 2006, 49, 1212–1216. [Google Scholar] [CrossRef]
- Mewshaw, R.E.; Marquis, K.L.; Shi, X.; McGaughey, G.; Stack, G.; Webb, M.B.; Abou-Gharbia, M.; Wasik, T.; Scerni, R.; Spangler, T. New generation dopaminergic agents 4. Exploiting the 2-methyl chroman scaffold. Synthesis and evaluation of two novel series of 2-(aminomethyl)-3, 4, 7, 9-tetrahydro-2H-pyrano [2, 3-e] indole and indol-8-one derivatives. Tetrahedron 1998, 54, 7081–7108. [Google Scholar] [CrossRef]
- Patil, N.T.; Yamamoto, Y. Coinage metal-assisted synthesis of heterocycles. Chem. Rev. 2008, 108, 3395–3442. [Google Scholar] [CrossRef]
- Alonso, F.; Yus, M.; Beletskaya, I. Non-conventional methodologies for transition-metal catalysed carbon-carbon coupling: A critical overview. Part 2: The Suzuki reaction. Tetrahedron 2008, 64, 3047–3101. [Google Scholar] [CrossRef]
- Doucet, H.; Hierso, J.C. Palladium-based catalytic systems for the synthesis of conjugated enynes by Sonogashira reactions and related alkynylations. Angew. Chem. Int. Ed. 2007, 46, 834–871. [Google Scholar] [CrossRef]
- Enthaler, S. Palladium-catalysed hydroxylation and alkoxylation. Chem. Soc. Rev. 2011, 40, 4912–4924. [Google Scholar] [CrossRef] [Green Version]
- Feldman, K.S.; Bruendl, M.M.; Schildknegt, K.; Bohnstedt, A.C. Inter-and intramolecular addition/cyclizations of sulfonamide anions with alkynyliodonium triflates. Synthesis of dihydropyrrole, pyrrole, indole, and tosylenamide heterocycles. J. Org. Chem. 1996, 61, 5440–5452. [Google Scholar] [CrossRef]
- Frisch, A.C.; Beller, M. Catalysts for cross-coupling reactions with non-activated alkyl halides. Angew. Chem. Int. Ed. 2005, 44, 674–688. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef] [Green Version]
- Kambe, N.; Iwasaki, T.; Terao, J. Pd-catalyzed cross-coupling reactions of alkyl halides. Chem. Soc. Rev. 2011, 40, 4937–4947. [Google Scholar] [CrossRef]
- Knappke, C.E.; von Wangelin, A.J. 35 years of palladium-catalyzed cross-coupling with Grignard reagents: How far have we come? Chem. Soc. Rev. 2011, 40, 4948–4962. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A. Efficient, selective, and recyclable palladium catalysts in carbon− carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Hiyama, T. Silicon-based cross-coupling reaction: An environmentally benign version. Chem. Soc. Rev. 2011, 40, 4893–4901. [Google Scholar] [CrossRef]
- Negishi, E.-I.; Anastasia, L. Palladium-catalyzed alkynylation. Chem. Rev. 2003, 103, 1979–2018. [Google Scholar] [CrossRef]
- Roglans, A.; Pla-Quintana, A.; Moreno-Manas, M. Diazonium salts as substrates in palladium-catalyzed cross-coupling reactions. Chem. Rev. 2006, 106, 4622–4643. [Google Scholar] [CrossRef] [PubMed]
- Rollet, P.; Kleist, W.; Dufaud, V.; Djakovitch, L. Copper-free heterogeneous catalysts for the Sonogashira cross-coupling reaction: Preparation, characterisation, activity and applications for organic synthesis. J. Mol. Catal. A Chem. 2005, 241, 39–51. [Google Scholar] [CrossRef]
- Selander, N.; Szabó, K.J. Catalysis by palladium pincer complexes. Chem. Rev. 2011, 111, 2048–2076. [Google Scholar] [CrossRef] [PubMed]
- Surry, D.S.; Buchwald, S.L. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 2008, 47, 6338–6361. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.E.; de Vries, J.G. Homogeneous catalysis for the production of fine chemicals. Palladium-and nickel-catalysed aromatic carbon–carbon bond formation. Top. Catal. 2002, 19, 111–118. [Google Scholar] [CrossRef]
- Zapf, A.; Beller, M. The development of efficient catalysts for palladium-catalyzed coupling reactions of aryl halides. Chem. Commun. 2005, 431–440. [Google Scholar] [CrossRef]
- Beletskaya, I.P. Palladium catalyzed CC and C-heteroatom bond formation reactions. Pure Appl. Chem. 1997, 69, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.F.; Anbarasan, P.; Neumann, H.; Beller, M. From noble metal to Nobel prize: Palladium-catalyzed coupling reactions as key methods in organic synthesis. Angew. Chem. Int. Ed. 2010, 49, 9047–9050. [Google Scholar] [CrossRef]
- Zeni, G.; Larock, R.C. Synthesis of heterocycles via palladium π-olefin and π-alkyne chemistry. Chem. Rev. 2004, 104, 2285–2310. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C—C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef]
- Fischer, E.; Jourdan, F. Ueber die hydrazine der brenztraubensäure. Ber. Dtsch. Chem. Ges. 1883, 16, 2241–2245. [Google Scholar] [CrossRef] [Green Version]
- Wagaw, S.; Yang, B.H.; Buchwald, S.L. A palladium-catalyzed strategy for the preparation of indoles: A novel entry into the Fischer indole synthesis. J. Am. Chem. Soc. 1998, 120, 6621–6622. [Google Scholar] [CrossRef]
- Gassman, P.G.; van Bergen, T.; Gruetzmacher, G. Use of halogen-sulfide complexes in the synthesis of indoles, oxindoles, and alkylated aromatic amines. J. Am. Chem. Soc. 1973, 95, 6508–6509. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Hillard, J.; Reddy, K.; Majumdar, K. A novel synthesis of indole derivatives via a claisen rearrangement. Tetrahedron Lett. 1974, 15, 1999–2002. [Google Scholar] [CrossRef]
- Baudin, J.-B.; Julia, S.A. Synthesis of indoles from N-aryl-1-alkenylsulphinamides. Tetrahedron Lett. 1986, 27, 837–840. [Google Scholar] [CrossRef]
- Houlihan, W.J.; Parrino, V.A.; Uike, Y. Lithiation of N-(2-alkylphenyl) alkanamides and related compounds. A modified Madelung indole synthesis. J. Org. Chem. 1981, 46, 4511–4515. [Google Scholar] [CrossRef]
- Mayadeo, M.; Gandhi, S. Convenient general synthesis of alhpha-aminolusaturated ketones, nitrilesaed their use in nenitzescue indole synthesis. J. Indian Chem. Soc. 1994, 71, 281–282. [Google Scholar]
- Bartoli, G.; Bosco, M.; Dalpozzo, R.; Palmieri, G.; Marcantoni, E. Reactivity of nitro- and nitroso-arenes with vinyl grignard reagents: Synthesis of 2-(trimethylsilyl)indoles. J. Chem. Soc. Perkin Trans. I 1991, 11, 2757–2761. [Google Scholar] [CrossRef]
- Bosco, M.; Dalpozzo, R.; Bartoli, G.; Palmieri, G.; Petrini, M. Mechanistic studies on the reaction of nitro-and nitrosoarenes with vinyl Grignard reagents. J. Chem. Soc. Perkin Trans. 2 1991, 5, 657–663. [Google Scholar] [CrossRef]
- Nenitzescu, C. Derivatives of 2-methyl-5-hydroxyindole. Bull. Soc. Chim. Rom. 1929, 11, 37–43. [Google Scholar]
- Bailey, W.F.; Jiang, X.-L. Preparation of Substituted Indolines via Anionic Cyclization. J. Org. Chem. 1996, 61, 2596–2597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liebeskind, L.S. A versatile synthesis of 3-substituted indolines and indoles. J. Org. Chem. 1996, 61, 2594–2595. [Google Scholar] [CrossRef] [PubMed]
- Bischler, A. Ueber die entstehung einiger substituirter indole. Ber. Dtsch. Chem. Ges. 1892, 25, 2860–2879. [Google Scholar] [CrossRef] [Green Version]
- Hemetsberger, H.; Knittel, D. Synthese und Thermolyse von α-Azidoacrylestern. Monatsh. Chem. 1972, 103, 194–204. [Google Scholar] [CrossRef]
- Molina, P.; Alcantara, J.; Lopez-Leonardo, C. Regiospecific intramolecular ring-closure of heterocumulene-substituted indoles: Formation of γ-carbolines and pyrimido [3, 4-a] indoles. Tetrahedron Lett. 1995, 36, 953–956. [Google Scholar] [CrossRef]
- Feldman, K.S.; Bruendl, M.M.; Schildknegt, K. Preparation of Five-Membered Nitrogen-Containing Heterocycles via [Three-Atom+ Two-Atom] Combination of Tosylamide Anions with Phenyl (propynyl) iodonium Triflate. J. Org. Chem. 1995, 60, 7722–7723. [Google Scholar] [CrossRef]
- Reissert, A. Einwirkung von oxalester und natriumäthylat auf nitrotoluole. synthese nitrirter phenylbrenztraubensäuren. Ber. Dtsch. Chem. Ges. 1897, 30, 1030–1053. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, T.; Chen, X.; Peng, G. A novel tin-mediated indole synthesis. J. Am. Chem. Soc. 1994, 116, 3127–3128. [Google Scholar] [CrossRef]
- Larock, R.C.; Yum, E.K. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. [Google Scholar] [CrossRef]
- Batcho, A.D.; Leimgruber, W. Indoles from 2-Methylnitrobenzenes by Condensation with Formamide Acetals Followed by Reduction: 4-Benzyloxyindole: 1H-Indole, 4-(phenylmethoxy)-. Org. Synth. 2003, 63, 214. [Google Scholar]
- Enders, D.; Grondal, C.; Huettl, M.R. Asymmetric organocatalytic domino reactions. Angew. Chem. Int. Ed. 2007, 46, 1570–1581. [Google Scholar] [CrossRef]
- Grigg, R.; Sridharan, V. Palladium catalysed cascade cyclisation-anion capture, relay switches and molecular queues. J. Organomet. Chem. 1999, 576, 65–87. [Google Scholar] [CrossRef]
- Malacria, M. Selective preparation of complex polycyclic molecules from acyclic precursors via radical mediated-or transition metal-catalyzed cascade reactions. Chem. Rev. 1996, 96, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Murakami, M. Formation of carbocycles through sequential carborhodation triggered by addition of organoborons. Chem. Commun. 2007, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J. Nickel-catalyzed reductive cyclizations and couplings. Angew. Chem. Int. Ed. 2004, 43, 3890–3908. [Google Scholar] [CrossRef] [PubMed]
- Negishi, E.-i.; Copéret, C.; Ma, S.; Liou, S.-Y.; Liu, F. Cyclic carbopalladation. A versatile synthetic methodology for the construction of cyclic organic compounds. Chem. Rev. 1996, 96, 365–394. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef]
- Nicolaou, K.; Montagnon, T.; Snyder, S.A. Tandem reactions, cascade sequences, and biomimetic strategies in total synthesis. Chem. Commun. 2003, 551–564. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, Q.; Yu, X.; Wu, J. Silver Triflate-Catalyzed or Electrophile-Mediated Tandem Reaction of N′-(2-Alkynylbenzylidene) hydrazides with Dimethyl Acetylenedicarboxylate. Adv. Synth. Catal. 2009, 351, 1692–1698. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Wu, J. AgOTf-catalyzed tandem reaction of N′-(2-alkynylbenzylidene) hydrazide with alkyne. Chem. Commun. 2009, 3469–3471. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, X.; Su, M.; Yang, X.; Wu, J. Multicatalytic Tandem Reactions of 2-Alkynylbenzaldoximes with Isocyanides. Adv. Synth. Catal. 2009, 351, 2702–2708. [Google Scholar] [CrossRef]
- Ding, Q.; He, X.; Wu, J. Synthesis of 2-aminobenzothiazole via copper (I)-catalyzed Tandem Reaction of 2-iodobenzenamine with isothiocyanate. J. Comb. Chem. 2009, 11, 587–591. [Google Scholar] [CrossRef]
- Ding, Q.; Huang, X.-G.; Wu, J. Facile synthesis of benzothiazoles via cascade reactions of 2-iodoanilines, acid chlorides and Lawesson’s reagent. J. Comb. Chem. 2009, 11, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Wang, Z.; Wu, J. Tandem Electrophilic Cyclization−[3+2] Cycloaddition− Rearrangement Reactions of 2-Alkynylbenzaldoxime, DMAD, and Br2. J. Org. Chem. 2009, 74, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Wang, Z.; Wu, J. Tandem cyclization-[3+ 3] cycloaddition reactions of 2-alkynylbenzaldoxime: Synthesis of fused 1, 2-dihydroisoquinolines. Tetrahedron Lett. 2009, 50, 198–200. [Google Scholar] [CrossRef]
- Ding, Q.; Wu, J. Access to Functionalized Isoquinoline N-Oxides via Sequential Electrophilic Cyclization/Cross-Coupling Reactions. Adv. Synth. Catal. 2008, 350, 1850–1854. [Google Scholar] [CrossRef]
- Ye, S.; Gao, K.; Wu, J. Three-Component Reactions of 2-Alkynylbenzaldoximes and α, β-Unsaturated Carbonyl Compounds with Bromine or Iodine Monochloride. Adv. Synth. Catal. 2010, 352, 1746–1751. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Z.; Yang, X.; Wu, J. Tandem Reactions of N′-(2-Alkynylbenzylidene) hydrazides with Silyl Enolates: A Facile Route to H-Pyrazolo [5, 1-a] isoquinolines. J. Comb. Chem. 2010, 12, 374–378. [Google Scholar] [CrossRef]
- Yu, X.; Wu, J. Synthesis of functionalized isoquinolines via sequential cyclization/cross-coupling reactions. J. Comb. Chem. 2009, 11, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, J. Synthesis of 1-(1 H-Indol-3-yl)-1, 2-dihydroisoquinolines via AgOTf-Catalyzed Three-Component Reactions of 2-Alkynylbenzaldehydes, Amines, and Indoles. J. Comb. Chem. 2010, 12, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kurti, L.; Czakó, B. Strategic Applications of Named Reactions in Organic Synthesis, ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Narasaka, K.; Kusama, H.; Yamashita, Y.; Sato, H. Org. React. Org. React. 35, 1, 1988. Chem. Lett. 1993, 1993, 489–492. [Google Scholar] [CrossRef]
- Qiu, G.; Ding, Q.; Ren, H.; Peng, Y.; Wu, J. Multicatalytic one-pot reaction of 1-(2-alkynylphenyl) ketoximes for generation of indole derivatives. Org. Lett. 2010, 12, 3975–3977. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Roussel, C.; Vanthuyne, N.; Piras, P. Atropisomerism and axial chirality in heteroaromatic compounds. Adv. Heterocycl. Chem. 2012, 105, 1–188. [Google Scholar]
- Ayitou, A.J.-L.; Jesuraj, J.L.; Barooah, N.; Ugrinov, A.; Sivaguru, J. Enantiospecific photochemical Norrish/Yang type II reaction of nonbiaryl atropchiral α-oxoamides in solution—Axial to point chirality transfer. J. Am. Chem. Soc. 2009, 131, 11314–11315. [Google Scholar] [CrossRef]
- Bock, L.; Adams, R. The stereochemistry of N-phenylpyrroles. The preparation and Resolution of N-2-Carboxyphenyl-2, 5-dimethyl-3-carboxypyrrole. XIII. J. Am. Chem. Soc. 1931, 53, 374–376. [Google Scholar] [CrossRef]
- Clayden, J. Tetrahedron Symposium-in-print on Axially Chiral Amides (Atropisomerism). Tetrahedron 2004, 60, 4325. [Google Scholar]
- Curran, D.P.; Qi, H.; Geib, S.J.; DeMello, N.C. Atroposelective thermal reactions of axially twisted amides and imides. J. Am. Chem. Soc. 1994, 116, 3131–3132. [Google Scholar] [CrossRef]
- Dai, X.; Wong, A.; Virgil, S.C. Synthesis and resolution of quinazolinone atropisomeric phosphine ligands. J. Org. Chem. 1998, 63, 2597–2600. [Google Scholar] [CrossRef]
- Hata, T.; Koide, H.; Taniguchi, N.; Uemura, M. Asymmetric Synthesis of Axially Chiral Anilides by Enantiotopic Lithiation of Tricarbonyl (N-methyl-N-acyl-2, 6-dimethylanilide) chromium Complex. Org. Lett. 2000, 2, 1907–1910. [Google Scholar] [CrossRef]
- Kawabata, T.; Jiang, C.; Hayashi, K.; Tsubaki, K.; Yoshimura, T.; Majumdar, S.; Sasamori, T.; Tokitoh, N. Axially Chiral Binaphthyl Surrogates with an Inner N− H− N Hydrogen Bond. J. Am. Chem. Soc. 2009, 131, 54–55. [Google Scholar] [CrossRef]
- Kawamoto, T.; Tomishima, M.; Yoneda, F.; Hayami, J.-I. Synthesis and reaction of novel 5-deazaflavins with axial chirality at pyrimidine ring moiety. Tetrahedron Lett. 1992, 33, 3169–3172. [Google Scholar] [CrossRef]
- Kitagawa, O.; Izawa, H.; Sato, K.; Dobashi, A.; Taguchi, T.; Shiro, M. Optically active axially chiral anilide and maleimide derivatives as new chiral reagents: Synthesis and application to asymmetric Diels−Alder reaction. J. Org. Chem. 1998, 63, 2634–2640. [Google Scholar] [CrossRef]
- Kondo, K.; Iida, T.; Fujita, H.; Suzuki, T.; Yamaguchi, K.; Murakami, Y. A Chiral Axis due to an Acyclic Imide–Ar Bond: A study of steric effects of Acyl groups on racemization. Tetrahedron 2000, 56, 8883–8891. [Google Scholar] [CrossRef]
- Mannschreck, A.; Koller, H.; Stuhler, G.; Davies, M.; Traber, J. The enantiomers of methaqualone and their unequal anticonvulsive activity. Eur. J. Med. Chem. 1984, 19, 381–383. [Google Scholar]
- Mino, T.; Komatsu, S.; Wakui, K.; Yamada, H.; Saotome, H.; Sakamoto, M.; Fujita, T. N-Aryl indole-derived C–N bond axially chiral phosphine ligands: Synthesis and application in palladium-catalyzed asymmetric allylic alkylation. Tetrahedron Asymmetry 2010, 21, 711–718. [Google Scholar] [CrossRef]
- Mintas, M.; Mihaljević, V.; Koller, H.; Schuster, D.; Mannschreck, A. Sterically hindered N-aryl-2 (1 H)-quinolones and N-aryl-6 (5 H)-phenanthridinones: Separation of enantiomers and barriers to racemization. J. Chem. Soc. Perkin Trans. 2 1990, 4, 619–624. [Google Scholar] [CrossRef]
- Roussel, C.; Adjimi, M.; Chemlal, A.; Djafri, A. Comparison of racemization processes in 1-arylpyrimidine-2-thione and 3-arylthiazoline-2-thione atropisomers and their oxygen analogs. J. Org. Chem. 1988, 53, 5076–5080. [Google Scholar] [CrossRef]
- Sakamoto, M.; Utsumi, N.; Ando, M.; Saeki, M.; Mino, T.; Fujita, T.; Katoh, A.; Nishio, T.; Kashima, C. Breaking the Symmetry of Axially Chiral N-Aryl-2 (1H)-pyrimidinones by Spontaneous Crystallization. Angew. Chem. Int. Ed. 2003, 42, 4360–4363. [Google Scholar] [CrossRef] [PubMed]
- Tokitoh, T.; Kobayashi, T.; Nakada, E.; Inoue, T.; Yokoshima, S.; Takahashi, H.; Natsugarib, H. Diastereoselective synthesis of atropisomeric 3-(2-substituted aryl) quinazolin-4-ones and their stereochemical properties. Heterocycles 2006, 70, 93–99. [Google Scholar]
- Tracey, M.R. Synthesis and Reactions of Ynamides and Allenamides; University of Minnesota: Minneapolis, MN, USA, 2005. [Google Scholar]
- Yılmaz, E.M.; Doğan, İ. Axially chiral N-(o-aryl)-2-thioxo-oxazolidine-4-one and rhodanine derivatives: Enantiomeric separation and determination of racemization barriers. Tetrahedron Asymmetry 2008, 19, 2184–2191. [Google Scholar] [CrossRef]
- Brandes, S.; Bella, M.; Kjærsgaard, A.; Jørgensen, K.A. Chirally Aminated 2-Naphthols—Organocatalytic synthesis of non-biaryl atropisomers by asymmetric Friedel–Crafts amination. Angew. Chem. 2006, 118, 1165–1169. [Google Scholar] [CrossRef]
- Clayden, J.; Turner, H. Enantiomerically enriched atropisomeric N, N′-diaryl ureas by oxidative kinetic resolution of their 2-sulfanyl derivatives. Tetrahedron Lett. 2009, 50, 3216–3219. [Google Scholar] [CrossRef]
- Di Iorio, N.; Righi, P.; Mazzanti, A.; Mancinelli, M.; Ciogli, A.; Bencivenni, G. Remote control of axial chirality: Aminocatalytic desymmetrization of N-arylmaleimides via vinylogous Michael addition. J. Am. Chem. Soc. 2014, 136, 10250–10253. [Google Scholar] [CrossRef]
- Duan, W.-L.; Imazaki, Y.; Shintani, R.; Hayashi, T. Asymmetric construction of chiral C–N axes through rhodium-catalyzed 1, 4-addition. Tetrahedron 2007, 63, 8529–8536. [Google Scholar] [CrossRef]
- Guo, R.; Li, K.-N.; Liu, B.; Zhu, H.-J.; Fan, Y.-M.; Gong, L.-Z. Asymmetric synthesis of heteroaryl atropisomers via a gold-catalyzed cycloisomerization–amination cascade reaction. Chem. Comm. 2014, 50, 5451–5454. [Google Scholar] [CrossRef] [Green Version]
- Kamikawa, K.; Arae, S.; Wu, W.Y.; Nakamura, C.; Takahashi, T.; Ogasawara, M. Simultaneous induction of axial and planar chirality in arene–chromium complexes by molybdenum-catalyzed enantioselective ring-closing metathesis. Chem. Eur. J. 2015, 21, 4954–4957. [Google Scholar] [CrossRef]
- Kitagawa, O.; Kohriyama, M.; Taguchi, T. Catalytic asymmetric synthesis of optically active atropisomeric anilides through enantioselective N-allylation with chiral Pd-tol-BINAP catalyst. J. Org. Chem. 2002, 67, 8682–8684. [Google Scholar] [CrossRef]
- Kitagawa, O.; Takahashi, M.; Yoshikawa, M.; Taguchi, T. Efficient synthesis of optically active atropisomeric anilides through catalytic asymmetric N-arylation reaction. J. Am. Chem. Soc. 2005, 127, 3676–3677. [Google Scholar] [CrossRef]
- Kitagawa, O.; Yoshikawa, M.; Tanabe, H.; Morita, T.; Takahashi, M.; Dobashi, Y.; Taguchi, T. Highly enantioselective synthesis of atropisomeric anilide derivatives through catalytic asymmetric N-arylation: Conformational analysis and application to asymmetric enolate chemistry. J. Am. Chem. Soc. 2006, 128, 12923–12931. [Google Scholar] [CrossRef]
- Liu, H.; Feng, W.; Kee, C.W.; Leow, D.; Loh, W.T.; Tan, C.H. Brønsted base-catalyzed tandem isomerization–Michael reactions of alkynes: Synthesis of oxacycles and azacycles. Adv. Synth. Catal. 2010, 352, 3373–3379. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Du, H. Asymmetric synthesis of axially chiral anilides by Pd-catalyzed allylic substitutions with P/olefin ligands. Org. Biomol. Chem. 2015, 13, 125–132. [Google Scholar] [CrossRef]
- Onodera, G.; Suto, M.; Takeuchi, R. Iridium-Catalyzed [2+ 2+ 2] Cycloaddition of α, ω-Diynes with Isocyanates. J. Org. Chem. 2012, 77, 908–920. [Google Scholar] [CrossRef]
- Oppenheimer, J.; Hsung, R.P.; Figueroa, R.; Johnson, W.L. Stereochemical control of both C− C and C− N axial chirality in the synthesis of chiral N, O-biaryls. Org. Lett. 2007, 9, 3969–3972. [Google Scholar] [CrossRef]
- Shirakawa, S.; Liu, K.; Maruoka, K. Catalytic asymmetric synthesis of axially chiral o-iodoanilides by phase-transfer catalyzed alkylations. J. Am. Chem. Soc. 2012, 134, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Morita, F.; Kusagaya, S.; Fukaya, H.; Kitagawa, O. Catalytic enantioselective synthesis of atropisomeric 2-aryl-4-quinolinone derivatives with an N–C chiral axis. Tetrahedron Asymmetry 2012, 23, 1657–1662. [Google Scholar] [CrossRef]
- Takahashi, I.; Suzuki, Y.; Kitagawa, O. Asymmetric Synthesis of Atropisomeric Compounds with an N‒C Chiral Axis. Org. Prep. Proced. Int. 2014, 46, 1–23. [Google Scholar] [CrossRef]
- Takahashi, M.; Kitagawa, O. Catalytic enantioselective synthesis of novel atropisomeric compounds having an NC chiral axis and their application to asymmetric reaction. J. Syn. Org. Chem. Jpn. 2011, 69, 985–993. [Google Scholar] [CrossRef]
- Takahashi, M.; Tanabe, H.; Nakamura, T.; Kuribara, D.; Yamazaki, T.; Kitagawa, O. Atropisomeric lactam chemistry: Catalytic enantioselective synthesis, application to asymmetric enolate chemistry and synthesis of key intermediates for NET inhibitors. Tetrahedron 2010, 66, 288–296. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, Y.; Suda, T.; Hirano, M. Synthesis of enantioenriched N-aryl-2-pyridones with chiral CN axes by rhodium-catalyzed [2+ 2+ 2] cycloaddition of alkynes with isocyanates. Synlett 2008, 2008, 1724–1728. [Google Scholar] [CrossRef]
- Tanaka, K.; Takeishi, K.; Noguchi, K. Enantioselective synthesis of axially chiral anilides through rhodium-catalyzed [2+ 2+ 2] cycloaddition of 1, 6-diynes with trimethylsilylynamides. J. Am. Chem. Soc. 2006, 128, 4586–4587. [Google Scholar] [CrossRef]
- Terauchi, J.; Curran, D.P. N-Allylation of anilides with chiral palladium catalysts: The first catalytic asymmetric synthesis of axially chiral anilides. Tetrahedron Asymmetry 2003, 14, 587–592. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2005, 105, 2873–2920. [Google Scholar] [CrossRef]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Annaka, K.; Fujita, A.; Sato, A.; Konakahara, T. InBr3-promoted divergent approach to polysubstituted indoles and quinolines from 2-ethynylanilines: Switch from an intramolecular cyclization to an intermolecular dimerization by a type of terminal substituent group. J. Org. Chem. 2008, 73, 4160–4165. [Google Scholar] [CrossRef]
- Taylor, E.C.; Katz, A.H.; Salgado-Zamora, H.; McKillop, A. Thallium in organic synthesis. 68. A convenient synthesis of 2-phenylindoles from anilides. Tetrahedron Lett. 1985, 26, 5963–5966. [Google Scholar] [CrossRef]
- Ototake, N.; Morimoto, Y.; Mokuya, A.; Fukaya, H.; Shida, Y.; Kitagawa, O. Catalytic Enantioselective Synthesis of Atropisomeric Indoles with an N—C Chiral Axis. Chem. Eur. J. 2010, 16, 6752–6755. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Shimizu, S.; Mokuya, A.; Ototake, N.; Saito, A.; Kitagawa, O. Enantioselective synthesis of N–C axially chiral indoles through chiral palladium-catalyzed 5-endo-hydroaminocyclization. Tetrahedron 2016, 72, 5221–5229. [Google Scholar] [CrossRef]
- Sakai, H.; Tsutsumi, K.; Morimoto, T.; Kakiuchi, K. One-Pot/Four-Step/Palladium-Catalyzed Synthesis of Indole Derivatives: The Combination of Heterogeneous and Homogeneous Systems. Adv. Synth. Catal. 2008, 350, 2498–2502. [Google Scholar] [CrossRef]
- Holenz, J.; Mercè, R.; Díaz, J.L.; Guitart, X.; Codony, X.; Dordal, A.; Romero, G.; Torrens, A.; Mas, J.; Andaluz, B. Medicinal chemistry driven approaches toward novel and selective serotonin 5-HT6 receptor ligands. J. Med. Chem. 2005, 48, 1781–1795. [Google Scholar] [CrossRef]
- Leclerc, V.; Yous, S.; Delagrange, P.; Boutin, J.A.; Renard, P.; Lesieur, D. Synthesis of nitroindole derivatives with high affinity and selectivity for melatoninergic binding sites MT3. J. Med. Chem. 2002, 45, 1853–1859. [Google Scholar] [CrossRef]
- Owa, T.; Okauchi, T.; Yoshimatsu, K.; Sugi, N.H.; Ozawa, Y.; Nagasu, T.; Koyanagi, N.; Okabe, T.; Kitoh, K.; Yoshino, H. A focused compound library of novel N-(7-indolyl) benzenesulfonamides for the discovery of potent cell cycle inhibitors. Bioorgan. Med. Chem. Lett. 2000, 10, 1223–1226. [Google Scholar] [CrossRef]
- Xu, Y.-C.; Johnson, K.W.; Phebus, L.A.; Cohen, M.; Nelson, D.L.; Schenck, K.; Walker, C.D.; Fritz, J.E.; Kaldor, S.W.; LeTourneau, M.E. N-[3-(2-Dimethylaminoethyl)-2-methyl-1 H-indol-5-yl]-4-fluorobenzamide: A Potent, Selective, and Orally Active 5-HT1F Receptor Agonist Potentially Useful for Migraine Therapy. J. Med. Chem. 2001, 44, 4031–4034. [Google Scholar] [CrossRef]
- Sanz, R.; Guilarte, V.; Pérez, A. Straightforward selective preparation of nitro-or amino-indoles from 2-halonitroanilines and alkynes. First synthesis of 7-amino-5-nitroindoles. Tetrahedron Lett. 2009, 50, 4423–4426. [Google Scholar] [CrossRef]
- Djakovitch, L.; Dufaud, V.; Zaidi, R. Heterogeneous Palladium Catalysts Applied to the Synthesis of 2-and 2, 3-Functionalised Indoles. Adv. Synth. Catal. 2006, 348, 715–724. [Google Scholar] [CrossRef]
- Dooleweerdt, K.; Ruhland, T.; Skrydstrup, T. Application of Ynamides in the Synthesis of 2-Amidoindoles. Org. Lett. 2009, 11, 221–224. [Google Scholar] [CrossRef]
- Fang, Y.-Q.; Lautens, M. A highly selective tandem cross-coupling of gem-dihaloolefins for a modular, efficient synthesis of highly functionalized indoles. J. Org. Chem. 2008, 73, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Pedersen, H.; Bang-Andersen, B.; Madsen, R.; Jørgensen, M. Palladium-Catalyzed Aryl Amination–Heck Cyclization Cascade: A One-Flask Approach to 3-Substituted Indoles. Angew. Chem. Int. Ed. 2008, 47, 888–890. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, J. Palladium-catalyzed, modular synthesis of highly functionalized indoles and tryptophans by direct annulation of substituted o-haloanilines and aldehydes. J. Org. Chem. 2006, 71, 7826–7834. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, M.; Zhang, Y.; Li, G.; Khodabocus, A.; Rodriguez, S.; Qu, B.; Farina, V.; Senanayake, C.H.; Lu, B.Z. A New entry to polycyclic indole skeletons via palladium-catalyzed intramolecular heteroannulation. Org. Lett. 2006, 8, 3573–3575. [Google Scholar] [CrossRef]
- Terrasson, V.; Michaux, J.; Gaucher, A.; Wehbe, J.; Marque, S.; Prim, D.; Campagne, J.M. Iron–Palladium Association in the Preparation of Indoles and One-Pot Synthesis of Bis (Indolyl) Methanes; Wiley Online Library: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zhao, J.; Larock, R.C. Synthesis of substituted carbazoles, indoles, and dibenzofurans by vinylic to aryl palladium migration. J. Org. Chem. 2006, 71, 5340–5348. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.L.; Koradin, C.; Dohle, W.; Knochel, P. Versatile indole synthesis by a 5-endo-dig cyclization mediated by potassium or cesium bases. Angew. Chem. Int. Ed. 2000, 39, 2488. [Google Scholar] [CrossRef]
- Dai, W.-M.; Sun, L.-P.; Guo, D.-S. Chemistry of aminophenols. Part 2: A general and efficient synthesis of indoles possessing a nitrogen substituent at the C4, C5, C6, and C7 positions. Tetrahedron Lett. 2002, 43, 7699–7702. [Google Scholar] [CrossRef]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-metal-catalyzed addition of heteroatom-hydrogen bonds to alkynes. Chem. Rev. 2004, 104, 3079–3160. [Google Scholar] [CrossRef]
- Chemler, S.R.; Fuller, P.H. Heterocycle synthesis by copper facilitated addition of heteroatoms to alkenes, alkynes and arenes. Chem. Soc. Rev. 2007, 36, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Hiroya, K.; Itoh, S.; Ozawa, M.; Kanamori, Y.; Sakamoto, T. Efficient construction of indole rings from 2-ethynylaniline derivatives catalyzed by copper (II) salts and its application to the tandem cyclization reactions. Tetrahedron Lett. 2002, 43, 1277–1280. [Google Scholar] [CrossRef]
- Lu, B.Z.; Zhao, W.; Wei, H.-X.; Dufour, M.; Farina, V.; Senanayake, C.H. A practical mild, one-pot, regiospecific synthesis of 2, 3-disubstituted indoles via consecutive Sonogashira and Cacchi reactions. Org. Lett. 2006, 8, 3271–3274. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Nakamura, I.; Yamamoto, Y. Intramolecular C− N Bond Addition of Amides to Alkynes Using Platinum Catalyst. J. Am. Chem. Soc. 2004, 126, 10546–10547. [Google Scholar] [CrossRef]
- Rao, R.M.; CH, U.R.; Mulakayala, N.; Alvala, M.; Arunasree, M.; Poondra, R.R.; Iqbal, J.; Pal, M. Sequential coupling/desilylation–coupling/cyclization in a single pot under Pd/C–Cu catalysis: Synthesis of 2-(hetero) aryl indoles. Org. Biomol. Chem. 2011, 9, 3808–3816. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ghosh, M.; Sarkar, P.; Ray, J.K. ZnCl2 and Pd/C catalyzed synthesis of 2-substituted indoles. Tetrahedron Lett. 2013, 54, 6691–6694. [Google Scholar] [CrossRef]
- Ahmed, A.; Ghosh, M.; Dhara, S.; Ray, J.K. A Mild Approach for the Synthesis of Indoles from N-(2-Iodo-aryl) formamides and Phenylacetylene by a Copper (I)-and Palladium-Catalyzed Cascade Process. Synlett 2014, 25, 2455–2458. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Manabe, K. One-pot synthesis of 2, 4-disubstituted indoles from N-tosyl-2, 3-dichloroaniline using palladium–dihydroxyterphenylphosphine catalyst. Org. Lett. 2014, 16, 2386–2389. [Google Scholar] [CrossRef]
- Bruneau, A.; Gustafson, K.P.; Yuan, N.; Tai, C.-W.; Persson, I.; Zou, X.; Bäckvall, J.-E. Synthesis of benzofurans and indoles from terminal alkynes and iodoaromatics catalyzed by recyclable palladium nanoparticles immobilized on siliceous mesocellular foam. Chem. Eur. J. 2017, 23, 12886–12891. [Google Scholar] [CrossRef] [PubMed]
- He, Y.P.; Wu, H.; Wang, Q.; Zhu, J. Palladium-Catalyzed Enantioselective Cacchi Reaction: Asymmetric Synthesis of Axially Chiral 2, 3-Disubstituted Indoles. Angew. Chem. 2020, 132, 2121–2125. [Google Scholar] [CrossRef]
- Chen, Y.; Markina, N.A.; Larock, R.C. An efficient, microwave-assisted, one-pot synthesis of indoles under Sonogashira conditions. Tetrahedron 2009, 65, 8908–8915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Perboni, A.; Sferrazza, A.; Stabile, P. 2, 3-Disubstituted indoles via palladium-catalyzed reaction of 2-alkynyltrifluoroacetanilides with arenediazonium tetrafluoroborates. Org. Lett. 2010, 12, 3279–3281. [Google Scholar] [CrossRef]
- Guilarte, V.; Castroviejo, M.P.; García-García, P.; Fernández-Rodríguez, M.A.; Sanz, R. Approaches to the synthesis of 2, 3-dihaloanilines. Useful precursors of 4-functionalized-1 H-indoles. J. Org. Chem. 2011, 76, 3416–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.Z.; Wei, H.-X.; Zhang, Y.; Zhao, W.; Dufour, M.; Li, G.; Farina, V.; Senanayake, C.H. One-pot and regiospecific synthesis of 2, 3-disubstituted indoles from 2-bromoanilides via consecutive palladium-catalyzed sonogashira coupling, amidopalladation, and reductive elimination. J. Org. Chem. 2013, 78, 4558–4562. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, H.; Meyer, J. Über neue organische phosphorverbindungen III. Phosphinmethylenderivate und phosphinimine. Helv. Chim. Acta 1919, 2, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhang, Z.; Zhou, Y.; Li, S.; Zhang, Y.; Wang, J. Palladium-Catalyzed Synthesis of Indoles and Isoquinolines with in Situ Generated Phosphinimine. J. Org. Chem. 2017, 82, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Minami, H.; Kanayama, T.; Tanaka, R.; Okamoto, N.; Sueda, T.; Yanada, R. Regioselective Arylative Ring-Closing Reaction of 2-Alkynylphenyl Derivatives: Formation of Arylated Benzoxazin-2-ones, Benzoxazin-2-amines and 2, 3-Disubstituted Indoles. Eur. J. Org. Chem. 2016, 2016, 5990–6000. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A.; Verdiglione, R. 2-(Aminomethyl)-3-arylindoles from 3-(o-Trifluoroacetamidoaryl)-1-propargylic alcohols, aryl Halides, and amines: A domino palladium-catalyzed three-component approach. Synthesis 2017, 49, 4163–4172. [Google Scholar]
- Arcadi, A.; Cianci, R.; Ferrara, G.; Marinelli, F. 3-(2-Alken-1-one-2-yl) indoles through the palladium-catalyzed reaction of 2-alkynyltrifluoroacetanilides with cyclic α-iodoenones. Tetrahedron 2010, 66, 2378–2383. [Google Scholar] [CrossRef]
- Álvarez, R.; Martínez, C.; Madich, Y.; Denis, J.G.; Aurrecoechea, J.M.; de Lera, Á.R. A General Synthesis of Alkenyl-Substituted Benzofurans, Indoles, and Isoquinolones by Cascade Palladium-Catalyzed Heterocyclization/Oxidative Heck Coupling. Chem. Eur. J. 2010, 16, 12746–12753. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Sferrazza, A. Heck reaction of arenediazonium salts with N, N-diprotected allylamines. Synthesis of cinnamylamines and indoles. Org. Biomol. Chem. 2011, 9, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, L.; Wu, X.; Jiang, H. Nucleopalladation triggering the oxidative heck reaction: A general strategy to diverse β-indole ketones. Org. Lett. 2013, 15, 5940–5943. [Google Scholar] [CrossRef]
- Janreddy, D.; Kavala, V.; Kuo, C.-W.; Kuo, T.-S.; He, C.-H.; Yao, C.-F. The PdCl2-catalyzed sequential heterocyclization/Michael addition cascade in the synthesis of 2, 3-disubstituted indoles. Tetrahedron 2013, 69, 3323–3330. [Google Scholar] [CrossRef]
- Reddy, V.; Vijaya Anand, R. Expedient access to unsymmetrical diarylindolylmethanes through palladium-catalyzed domino electrophilic cyclization–extended conjugate addition approach. Org. Lett. 2015, 17, 3390–3393. [Google Scholar] [CrossRef]
- Chen, J.; Han, X.; Lu, X. Enantioselective Synthesis of Tetrahydropyrano [3, 4-b] indoles: Palladium (II)-Catalyzed Aminopalladation/1, 4-Addition Sequence. Angew. Chem. 2017, 129, 14890–14893. [Google Scholar] [CrossRef]
- Tang, S.; Xie, Y.-X.; Li, J.-H.; Wang, N.-X. PdX2/CuX2-Catalyzed annulation of 2-Ethynylbenzeneamines: Selective synthesis of 2-Substituted 3-Halo-1H-indoles. Synthesis 2007, 2007, 1841–1847. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Yu, S.-C.; Liang, Y.; Peng, P.; Tang, B.-X.; Li, J.-H. Halopalladation Cyclization of Alkynes with Azides: Selective Synthesis of 4-Haloisoquinolines and 3-Haloindoles. Synlett 2011, 2011, 982–988. [Google Scholar]
- Han, X.; Lu, X. Cationic Pd (II)-Catalyzed Tandem Reaction of 2-Arylethynylanilines and Aldehydes: An Efficient Synthesis of Substituted 3-Hydroxymethyl Indoles. Org. Lett. 2010, 12, 3336–3339. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, D.; Nian, Y.; Zhang, L.; Yang, W.; Liu, H. Palladium-catalyzed difunctionalization of alkynes via C–N and S–N cleavages: A versatile approach to highly functional indoles. Org. Lett. 2014, 16, 5124–5127. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Veltri, L.; Salerno, G.; Mancuso, R.; Costa, M. Multicomponent Cascade Reactions: A novel and expedient approach to functionalized Indoles by an unprecedented nucleophilic addition-heterocyclization-oxidative alkoxycarbonylation sequence. Adv. Synth. Catal. 2010, 352, 3355–3363. [Google Scholar] [CrossRef]
- Gabriele, B.; Veltri, L.; Mancuso, R.; Salerno, G.; Costa, M. A general synthesis of Indole-3-carboxylic esters by Palladium-catalyzed direct oxidative carbonylation of 2-Alkynylaniline derivatives. Eur. J. Org. Chem. 2012, 2012, 2549–2559. [Google Scholar] [CrossRef]
- Chen, J.; Han, X.; Lu, X. Atom-economic synthesis of pentaleno [2, 1-b] indoles via Tandem cyclization of alkynones initiated by aminopalladation. J. Org. Chem. 2017, 82, 1977–1985. [Google Scholar] [CrossRef]
- Xia, X.-F.; Zhang, L.-L.; Song, X.-R.; Niu, Y.-N.; Liu, X.-Y.; Liang, Y.-M. Palladium–copper-cocatalyzed intramolecular oxidative coupling: An efficient and atom-economical strategy for the synthesis of 3-acylindoles. Chem. Comm. 2013, 49, 1410–1412. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Lyu, C.; Yong, W.; Li, J.; Zhu, X.; Rao, W. Synthesis of 1 H-indole-3-sulfonates via palladium-catalyzed tandem reactions of 2-alkynyl arylazides with sulfonic acids. Org. Biomol. Chem. 2017, 15, 6080–6083. [Google Scholar] [CrossRef]
- Qiu, G.; Chen, C.; Yao, L.; Wu, J. An efficient route to 3-Amidylindoles via a Palladium-Catalyzed tandem reaction of 2-alkynylanilines with isocyanides. Adv. Synth. Catal. 2013, 355, 1579–1584. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, J.; Liang, D.; Zhu, Q. Synthesis of 1H-Indole-3-carboxamidines through a Palladium-Catalyzed Three-Component Reaction Involving Isocyanide Insertion as a Key Step. Adv. Synth. Catal. 2013, 355, 3290–3294. [Google Scholar] [CrossRef]
- Qiu, G.; Qiu, X.; Liu, J.; Wu, J. Switchable Synthesis of 3-Cyanoindoles and 3-Amidylindoles via a Palladium-Catalyzed Reaction of N, N-Dimethyl-2-alkynylaniline with Isocyanide. Adv. Synth. Catal. 2013, 355, 2441–2446. [Google Scholar] [CrossRef]
- Hu, Z.; Luo, S.; Zhu, Q. Aminopalladation-Triggered Carbene Insertion Reaction: Synthesis of 2-(1H-Indol-3-yl) acetates. Adv. Synth. Catal. 2015, 357, 1060–1064. [Google Scholar] [CrossRef]
- Yao, B.; Wang, Q.; Zhu, J. Palladium-Catalyzed Coupling of ortho-Alkynylanilines with Terminal Alkynes Under Aerobic Conditions: Efficient Synthesis of 2, 3-Disubstituted 3-Alkynylindoles. Angew. Chem. Int. Ed. 2012, 51, 12311–12315. [Google Scholar] [CrossRef]
- Yao, B.; Wang, Q.; Zhu, J. Pd/C-Catalyzed Cyclizative Cross-Coupling of Two ortho-Alkynylanilines under Aerobic Conditions: Synthesis of 2, 3′-Bisindoles. Chem. Eur. J. 2015, 21, 7413–7416. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Macdonald, S.J.; Harrity, J.P. A borylative cyclisation towards indole boronic esters. Chem. Comm. 2010, 46, 8770–8772. [Google Scholar] [CrossRef]
- Guo, Y.J.; Tang, R.Y.; Li, J.H.; Zhong, P.; Zhang, X.G. Palladium-Catalyzed Annulation of 2-(1-Alkynyl) benzenamines with Disulfides: Synthesis of 3-Sulfenylindoles. Adv. Synth. Catal. 2009, 351, 2615–2618. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Huang, L.; Chen, H.; Jiang, H. Highly efficient and practical synthesis of functionalized 1, 5-dienes via Pd (II)-catalyzed halohomoallylation of alkynes. RSC Adv. 2013, 3, 11529–11532. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Jiang, H.; Wu, W.; Zhao, J. Palladium-catalyzed coupling of alkynes with unactivated alkenes in ionic liquids: A regio-and stereoselective synthesis of functionalized 1, 6-dienes and their analogues. J. Org. Chem. 2013, 78, 12477–12486. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Wu, W.; Jiang, H. Novel palladium-catalyzed cascade carboxylative annulation to construct functionalized γ-lactones in ionic liquids. Chem. Comm. 2014, 50, 1381–1383. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yang, S.; Wu, W.; Qi, C.; Deng, Z.; Jiang, H. Synthesis of 1, 4-dienes by Pd (II)-catalyzed haloallylation of alkynes with allylic alcohols in ionic liquids. Tetrahedron 2014, 70, 1516–1523. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Yang, S.; Huang, L.; Wu, W.; Sun, Y.; Jiang, H. Palladium-Catalyzed Cascade Annulation To Construct Functionalized β-and γ-Lactones in Ionic Liquids. Angew. Chem. Int. Ed. 2014, 53, 7219–7222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Z.; Yang, S.; Zhang, Z.; Wu, W.; Jiang, H. Palladium-catalyzed tandem annulation: A strategy to construct 2, 3-difunctionalized benzofuran derivatives in ionic liquids. J. Org. Chem. 2015, 80, 3870–3879. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, H. Haloalkynes: A powerful and versatile building block in organic synthesis. Acc. Chem. Res. 2014, 47, 2483–2504. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-R.; Jiang, H.-F.; Li, Y.-Q.; Chen, H.-J.; Luo, W.; Xu, Y.-B. Protonolysis of the carbon–palladium bond in palladium (II)-catalyzed enyne cyclization in imidazolium-type ionic liquids. Tetrahedron 2008, 64, 2930–2937. [Google Scholar] [CrossRef]
- Chaplin, J.H.; Flynn, B.L. A multi-component coupling approach to benzo [b] furans and indolesElectronic supplementary information (ESI) available: Experimental procedures and spectroscopic data for 14a–h, 15a and 16. Chem. Commun. 2001, 1594–1595. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, W.; Jiang, Z.-j.; Wang, K.; Zheng, X.-l.; Fu, H.-y.; Chen, H.; Li, R.-X. One-pot synthesis of 2-substituted benzo [b] furans via Pd–tetraphosphine catalyzed coupling of 2-halophenols with alkynes. Chem. Comm. 2014, 50, 6023–6026. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Y.; Chu, L.; Wang, R.W.; Zhang, X.; Qing, F.L. Copper-Catalyzed Oxidative Trifluoromethylthiolation of Aryl Boronic Acids with TMSCF3 and Elemental Sulfur. Angew. Chem. 2012, 124, 2542–2545. [Google Scholar] [CrossRef]
- Chu, L.; Qing, F.-L. Copper-mediated oxidative trifluoromethylation of boronic acids. Org. Lett. 2010, 12, 5060–5063. [Google Scholar] [CrossRef]
- Helton, M.E.; Chen, P.; Paul, P.P.; Tyeklár, Z.; Sommer, R.D.; Zakharov, L.N.; Rheingold, A.L.; Solomon, E.I.; Karlin, K.D. Reaction of elemental sulfur with a copper (I) complex forming a trans-μ-1, 2 end-on disulfide complex: New directions in copper− sulfur chemistry. J. Am. Chem. Soc. 2003, 125, 1160–1161. [Google Scholar] [CrossRef] [PubMed]
- Posner, G. Direct and convenient preparation of lithium phenylthio (alkyl) cuprate reagents. Synthesis 1974, 662–663. [Google Scholar] [CrossRef]
- Senecal, T.D.; Parsons, A.T.; Buchwald, S.L. Room temperature aryl trifluoromethylation via copper-mediated oxidative cross-coupling. J. Org. Chem. 2011, 76, 1174–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. Assembly of 3-sulfenylbenzofurans and 3-sulfenylindoles by palladium-catalyzed cascade annulation/arylthiolation reaction. J. Org. Chem. 2016, 81, 2875–2887. [Google Scholar] [CrossRef]
- Sheng, J.; Li, S.; Wu, J. Synthesis of 3-((trifluoromethyl) thio) indoles via a reaction of 2-alkynylaniline with trifluoromethanesulfanylamide. Chem. Comm. 2014, 50, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, F.; Du, Y.; Zhao, L.; Chen, L.; Wang, J.; Liu, H. Highly selective intramolecular addition of C–N and S–N bonds to alkynes catalyzed by palladium: A practical access to two distinct functional indoles. RSC Adv. 2016, 6, 70682–70690. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G.; Lupinacci, E.; Ruffolo, G.; Costa, M. Versatile synthesis of quinoline-3-carboxylic esters and indol-2-acetic esters by palladium-catalyzed carbonylation of 1-(2-aminoaryl)-2-yn-1-ols. J. Org. Chem. 2008, 73, 4971–4977. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient synthesis of ureas by direct palladium-catalyzed oxidative carbonylation of amines. J. Org. Chem. 2004, 69, 4741–4750. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kawamatsu, H.; Sonoda, N. A facile method for the synthesis of thiocarbamates: Palladium-catalyzed reaction of disulfide, amine, and carbon monoxide. J. Org. Chem. 2005, 70, 2551–2554. [Google Scholar] [CrossRef] [PubMed]
- Cacchi, S.; Fabrizi, G.; Filisti, E. Palladium-catalyzed synthesis of free-NH indole 2-acetamides and derivatives from ethyl 3-(o-trifluoroacetamidoaryl)-1-propargylic carbonates. Synlett 2009, 2009, 1817–1821. [Google Scholar] [CrossRef]
- Ambrogio, I.; Cacchi, S.; Fabrizi, G.; Prastaro, A. 3-(o-Trifluoroacetamidoaryl)-1-propargylic esters: Common intermediates for the palladium-catalyzed synthesis of 2-aminomethyl-, 2-vinylic, and 2-alkylindoles. Tetrahedron 2009, 65, 8916–8929. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Molinaro, C.; Verdiglione, R. Palladium-catalyzed synthesis of 2-(aminomethyl) indoles from 3-(o-trifluoroacetamidoaryl)-1-propargylic alcohols and amines. J. Org. Chem. 2014, 79, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, N.; Babu, M.H.; Dwivedi, V.; Kant, R.; Reddy, M.S. Palladium-Catalyzed Tandem Intramolecular Oxy/Amino-Palladation/Isocyanide Insertion: Synthesis of α-Benzofuranyl/Indolylacetamides. Org. Lett. 2014, 16, 2908–2911. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Song, H.; Liu, Y.; Wang, Q. Regio-and Chemoselective N-1 Acylation of Indoles: Pd-Catalyzed Domino Cyclization to Afford 1, 2-Fused Tricyclic Indole Scaffolds. Chem. Eur. J. 2015, 21, 5337–5340. [Google Scholar] [CrossRef]
- Das, B.; Kundu, P.; Chowdhury, C. Facile synthesis of 2-arylmethylindoles and 2-vinylic indoles through palladium-catalyzed heteroannulations of 2-(2-propynyl) aniline and 2-(2-propynyl) tosylanilide. Org. Biomol. Chem. 2014, 12, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, C.; Das, B.; Mukherjee, S.; Achari, B. Palladium-catalyzed approach for the general synthesis of (E)-2-arylmethylidene-N-tosylindolines and (E)-2-arylmethylidene-N-tosyl/nosyltetrahydroquinolines: Access to 2-substituted indoles and quinolines. J. Org. Chem. 2012, 77, 5108–5119. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, J.; Fu, Y.; Liu, L.; Guo, Q.-X. Regioselective Pd-catalyzed indolization of 2-bromoanilines with internal alkynes using phosphine-free ligands. Tetrahedron Lett. 2008, 49, 3458–3462. [Google Scholar] [CrossRef]
- Batail, N.; Bendjeriou, A.; Lomberget, T.; Barret, R.; Dufaud, V.; Djakovitch, L. First Heterogeneous Ligand-and Salt-Free Larock Indole Synthesis. Adv. Synth. Catal. 2009, 351, 2055–2062. [Google Scholar] [CrossRef]
- Batail, N.; Dufaud, V.; Djakovitch, L. Larock heteroannulation of 2-bromoanilines with internal alkynes via ligand and salt free Pd/C catalysed reaction. Tetrahedron Lett. 2011, 52, 1916–1918. [Google Scholar] [CrossRef]
- Monguchi, Y.; Mori, S.; Aoyagi, S.; Tsutsui, A.; Maegawa, T.; Sajiki, H. Palladium on carbon-catalyzed synthesis of 2-and 2, 3-substituted indoles under heterogeneous conditions. Org. Biomol. Chem. 2010, 8, 3338–3342. [Google Scholar] [CrossRef]
- Ang, W.J.; Tai, C.-H.; Lo, L.-C.; Lam, Y. Palladium-catalyzed annulation of internal alkynes in aqueous medium. RSC Adv. 2014, 4, 4921–4929. [Google Scholar] [CrossRef]
- Denmark, S.E.; Baird, J.D. Preparation of 2, 3-disubstituted indoles by sequential Larock heteroannulation and silicon-based cross-coupling reactions. Tetrahedron 2009, 65, 3120–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danner, P.; Morkunas, M.; Maier, M.E. Synthesis of d-Abrines by Palladium-catalyzed Reaction of ortho-Iodoanilines with N-Boc-N-methylalanyl-substituted Acetaldehyde and Acetylene. Org. Lett. 2013, 15, 2474–2477. [Google Scholar] [CrossRef] [Green Version]
- Kondoh, A.; Yorimitsu, H.; Oshima, K. Synthesis of 2-indolylphosphines by palladium-catalyzed annulation of 1-alkynylphosphine sulfides with 2-iodoanilines. Org. Lett. 2010, 12, 1476–1479. [Google Scholar] [CrossRef]
- Goswami, K.; Duttagupta, I.; Sinha, S. Synthesis of optically active 2-and 3-indolylglycine derivatives and their oxygen analogues. J. Org. Chem. 2012, 77, 7081–7085. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Geng, W.; Zhang, W.X.; Xi, Z. Palladium-catalyzed one-pot three-or four-component coupling of aryl iodides, alkynes, and amines through C—N bond cleavage: Efficient synthesis of indole derivatives. Chem. Eur. J. 2014, 20, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wei, J.; Geng, W.; Zhang, W.X.; Xi, Z. Transfer of Aryl Halide to Alkyl Halide: Reductive elimination of alkylhalide from alkylpalladium halides containing syn-β-Hydrogen atoms. Angew. Chem. 2014, 126, 14761–14765. [Google Scholar] [CrossRef]
- Hao, W.; Wang, H.; Walsh, P.J.; Xi, Z. Combining Pd (π-allyl) Cp and PPh 3 as a unique catalyst for efficient synthesis of alkyliodo indoles via C (sp 3)–I reductive elimination. Org. Chem. Front. 2015, 2, 1080–1084. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, S. Coupling and cyclization of o-iodoanilines and propargylic bromides via allenes: An efficient entry to indomethacin. Org. Lett. 2013, 15, 2782–2785. [Google Scholar] [CrossRef]
- Panyam, P.K.R.; Gandhi, T. Palladium (II)/N-heterocyclic carbene-catalyzed regioselective heteroannulation of tertiary propargyl alcohols and o-Haloanilines to form 2-Alkenylindoles. Adv. Synth. Catal. 2017, 359, 1144–1151. [Google Scholar] [CrossRef]
- Onishi, K.; Oikawa, K.; Yano, H.; Suzuki, T.; Obora, Y. N, N-Dimethylformamide-stabilized palladium nanoclusters as a catalyst for Larock indole synthesis. RSC Adv. 2018, 8, 11324–11329. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, H.-Y.; Luo, Y.; Chen, C.; Cao, Y.; Chen, P.; Guo, Y.-L.; Lan, Y.; Liu, G. Regioselective palladium-catalyzed CH Bond trifluoroethylation of indoles: Exploration and mechanistic insight. ACS Catal. 2018, 8, 2173–2180. [Google Scholar] [CrossRef]
- Alberico, D.; Scott, M.E.; Lautens, M. Aryl− aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 2007, 107, 174–238. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Wang, N.; Jin, J.; Lu, P.; Wang, Y. Palladium-Catalyzed reaction of arylamine and diarylacetylene: Solvent-controlled construction of 2, 3-diarylindoles and pentaarylpyrroles. Eur. J. Org. Chem. 2012, 2012, 4380–4386. [Google Scholar] [CrossRef]
- Shen, D.; Han, J.; Chen, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Mild and efficient one-pot synthesis of 2-(perfluoroalkyl) indoles by means of sequential Michael-type addition and Pd (II)-catalyzed cross-dehydrogenative coupling (CDC) reaction. Org. Lett. 2015, 17, 3283–3285. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, C.; Li, S.; Pan, D.; Ding, S.; Cui, Y.; Jiao, N. Indoles from simple anilines and alkynes: Palladium-catalyzed C H activation using dioxygen as the oxidant. Angew. Chem. 2009, 121, 4642–4646. [Google Scholar] [CrossRef]
- Nakamura, I.; Nemoto, T.; Shiraiwa, N.; Terada, M. Palladium-catalyzed indolization of N-aroylbenzotriazoles with disubstituted alkynes. Org. Lett. 2009, 11, 1055–1058. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Kirichenko, K. Acyl anion synthons: Benzotriazole stabilized compared to classical. Arkivoc 2006, 4, 119–151. [Google Scholar] [CrossRef] [Green Version]
- Katritzky, A.R.; Lan, X.; Yang, J.Z.; Denisko, O.V. Properties and synthetic utility of N-substituted benzotriazoles. Chem. Rev. 1998, 98, 409–548. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Suzuki, K.; Wang, Z. Acylbenzotriazoles as advantageous N-, C-, S-, and O-acylating agents. Synlett 2005, 2005, 1656–1665. [Google Scholar] [CrossRef]
- Solé, D.; Vallverdú, L.; Solans, X.; Font-Bardía, M.; Bonjoch, J. Intramolecular Pd-mediated processes of amino-tethered aryl halides and ketones: Insight into the ketone α-arylation and carbonyl-addition dichotomy. A new class of four-membered azapalladacycles. J. Am. Chem. Soc. 2003, 125, 1587–1594. [Google Scholar] [CrossRef]
- Horino, H.; Inoue, N. Ortho vinylation of aromatic amides via cyclopalladation complexes. J. Org. Chem. 1981, 46, 4416–4422. [Google Scholar] [CrossRef]
- Tremont, S.J.; Rahman, H.U. Ortho-alkylation of acetanilides using alkyl halides and palladium acetate. J. Am. Chem. Soc. 1984, 106, 5759–5760. [Google Scholar] [CrossRef]
- Zhou, F.; Han, X.; Lu, X. Synthesis of indoles via palladium-catalyzed C–H activation of N-aryl amides followed by coupling with alkynes. Tetrahedron Lett. 2011, 52, 4681–4685. [Google Scholar] [CrossRef]
- Chen, J.; Pang, Q.; Sun, Y.; Li, X. Synthesis of N-(2-pyridyl) indoles via Pd (II)-catalyzed oxidative coupling. J. Org. Chem. 2011, 76, 3523–3526. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, L.; Natte, K.; Neumann, H.; Beller, M.; Wu, X.F. Palladium@ Cerium (IV) Oxide-Catalyzed Oxidative Synthesis of N-(2-Pyridyl) indoles via C—H Activation Reaction. Adv. Synth. Catal. 2014, 356, 2955–2959. [Google Scholar] [CrossRef]
- Sanz, R.; Castroviejo, M.P.; Guilarte, V.; Pérez, A.; Fananas, F.J. Regioselective Synthesis of 4-and 7-alkoxyindoles from 2, 3-dihalophenols: Application to the preparation of indole inhibitors of phospholipase A2. J. Org. Chem. 2007, 72, 5113–5118. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.; Castroviejo, M.P.; Fernández, Y.; Fañanás, F.J. A new and efficient synthesis of 4-functionalized benzo [b] furans from 2, 3-dihalophenols. J. Org. Chem. 2005, 70, 6548–6551. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Sandmann, R.; Kondrashov, M.V. Thieme Chemistry Journal Awardees-Where Are They Now? Palladium-Catalyzed N-Arylation-Hydroamination Sequence for the Synthesis of Indoles with Sterically Demanding N-Substituents. Synlett 2009, 2009, 1219–1222. [Google Scholar] [CrossRef]
- Ackermann, L.; Sandmann, R.; Schinkel, M.; Kondrashov, M.V. Palladium-catalyzed sequential indole synthesis using sterically hindered amines. Tetrahedron 2009, 65, 8930–8939. [Google Scholar] [CrossRef]
- Liang, Y.; Meng, T.; Zhang, H.-J.; Xi, Z. Palladium-catalyzed, one-pot, three-component approach to α-alkynyl indoles from o-bromo-(2, 2-dibromovinyl) benzenes, terminal alkynes and arylamines. Synlett 2011, 2011, 911–914. [Google Scholar] [CrossRef]
- Alsabeh, P.G.; Lundgren, R.J.; Longobardi, L.E.; Stradiotto, M. Palladium-catalyzed synthesis of indoles via ammonia cross-coupling–alkyne cyclization. Chem. Comm. 2011, 47, 6936–6938. [Google Scholar] [CrossRef]
- Lavery, C.B.; McDonald, R.; Stradiotto, M. Efficient palladium-catalyzed synthesis of substituted indoles employing a new (silanyloxyphenyl) phosphine ligand. Chem. Comm. 2012, 48, 7277–7279. [Google Scholar] [CrossRef]
- Halland, N.; Nazaré, M.; Alonso, J.; R’kyek, O.; Lindenschmidt, A. A general and mild domino approach to substituted 1-aminoindoles. Chem. Comm. 2011, 47, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Dibakar, M.; Selvakumar, K.; Ruckmani, K.; Sivakumar, M. Efficient indoles and anilines syntheses employing tert-butyl sulfinamide as ammonia surrogate. Tetrahedron Lett. 2011, 52, 5625–5628. [Google Scholar] [CrossRef]
- Yao, P.-Y.; Zhang, Y.; Hsung, R.P.; Zhao, K. A sequential metal-catalyzed C− N bond formation in the synthesis of 2-amido-indoles. Org. Lett. 2008, 10, 4275–4278. [Google Scholar] [CrossRef] [PubMed] [Green Version]














































































































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayesha; Bilal, M.; Rasool, N.; Khan, S.G.; Rashid, U.; Altaf, H.; Ali, I. Synthesis of Indoles via Intermolecular and Intramolecular Cyclization by Using Palladium-Based Catalysts. Catalysts 2021, 11, 1018. https://doi.org/10.3390/catal11091018
Ayesha, Bilal M, Rasool N, Khan SG, Rashid U, Altaf H, Ali I. Synthesis of Indoles via Intermolecular and Intramolecular Cyclization by Using Palladium-Based Catalysts. Catalysts. 2021; 11(9):1018. https://doi.org/10.3390/catal11091018
Chicago/Turabian StyleAyesha, Muhammad Bilal, Nasir Rasool, Samreen Gul Khan, Umer Rashid, Humaira Altaf, and Imtiaz Ali. 2021. "Synthesis of Indoles via Intermolecular and Intramolecular Cyclization by Using Palladium-Based Catalysts" Catalysts 11, no. 9: 1018. https://doi.org/10.3390/catal11091018
APA StyleAyesha, Bilal, M., Rasool, N., Khan, S. G., Rashid, U., Altaf, H., & Ali, I. (2021). Synthesis of Indoles via Intermolecular and Intramolecular Cyclization by Using Palladium-Based Catalysts. Catalysts, 11(9), 1018. https://doi.org/10.3390/catal11091018