Phosphate Enrichment of Niobium-Based Catalytic Surfaces in Relation to Reactions of Carbohydrate Biomass Conversion: The Case Studies of Inulin Hydrolysis and Fructose Dehydration
Abstract
:1. Introduction
2. Results
2.1. Mixture Preparation and Characterization
2.2. Characterization of Surface Acidity
2.2.1. Intrinsic and Effective Acidity by Liquid–Solid Phase Titrations
2.2.2. Acid Site Intrinsic and Effective Nature
2.3. Catalytic Activity
2.3.1. Catalytic Inulin Hydrolysis
2.3.2. Catalytic Dehydration of Fructose/Glucose
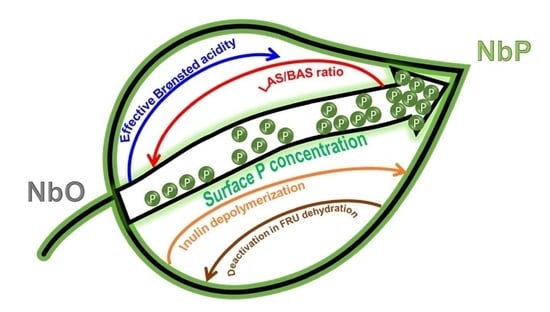
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation and Characterization
4.3. Characterization of Surface Acidity
4.3.1. Acid Site Intrinsic Nature and Water Tolerance Properties
4.3.2. Liquid–Solid Acid Site Titration for the Determination of Intrinsic and Effective Acidity
4.4. Catalytic Experiments and Analysis
4.4.1. Catalytic Tests of Inulin Hydrolysis
4.4.2. Catalytic Tests of Fructose and Glucose Dehydration
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Experimental Details
Reagents and Materials
Appendix A.2. Solid–Liquid Phase Titration of Surface Acid Sites by Phenylethylamine Probe
Appendix A.3. Kinetic Interpretation of Catalytic Data
Inulin Hydrolysis
References
- Ziolek, M.; Sobczak, I. The role of niobium component in heterogeneous catalysts. Catal. Today 2017, 285, 211–225. [Google Scholar] [CrossRef]
- Ziolek, M. Niobium-containing catalysts—The state of the art. Catal. Today 2003, 78, 47–64. [Google Scholar] [CrossRef]
- Tanabe, K. Catalytic application of niobium compounds. Catal. Today 2003, 78, 65–77. [Google Scholar] [CrossRef]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef]
- Iizuka, T.; Ogasawara, K.; Tanabe, K. Acidic and Catalytic Properties of Niobium Pentaoxide. Bull. Chem. Soc. Jpn. 1983, 56, 2927–2931. [Google Scholar] [CrossRef]
- Védrine, J.C.; Coudurier, G.; Ouqour, A.; Pries De Oliveira, P.G.; Volta, J.C. Niobium oxide based materials as catalysts for acidic and partial oxidation type reactions. Catal. Today 1996, 28, 3–15. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Y. Solid-State NMR study of dehydration of layered α-niobium phosphate. Inorg. Chem. 2009, 48, 10186–10192. [Google Scholar] [CrossRef]
- Okazaki, S.; Wada, N. Surface properties and catalytic activities of amorphous niobium phosphate and a comparison with those of H3PO4-Treated niobium oxide. Catal. Today 1993, 16, 349–359. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Biella, S.; Auroux, A. Intrinsic and Effective Acidity Study of Niobic Acid and Niobium Phosphate by a Multitechnique Approach. Chem. Mater. 2005, 17, 6128–6136. [Google Scholar] [CrossRef]
- Sun, Q.; Auroux, A.; Shen, J. Surface acidity of niobium phosphate and steam reforming of dimethoxymethane over CuZnO/Al2O3-NbP complex catalysts. J. Catal. 2006, 244, 1–9. [Google Scholar] [CrossRef]
- Martins, R.L.; Schitine, W.J.; Castro, F.R. Texture, surface acidic and catalytic properties of niobium phosphate. Catal. Today 1989, 5, 483–491. [Google Scholar] [CrossRef]
- Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Nb2O5 nH2O as a Heterogeneous Catalyst with Water-Tolerant Lewis Acid Sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, T.; Busca, G.; Carlini, C.; Giuttari, M.; Raspolli Galletti, A.M.; Sbrana, G. Acid sites characterization of niobium phosphate catalysts and their activity in fructose dehydration to 5-hydroxymethyl-2-furaldehyde. J. Mol. Catal. A Chem. 2000, 151, 233–243. [Google Scholar] [CrossRef]
- Vieira, J.L.; Paul, G.; Iga, G.D.; Cabral, N.M.; Bueno, J.M.C.; Bisio, C.; Gallo, J.M.R. Niobium phosphates as bifunctional catalysts for the conversion of biomass-derived monosaccharides. Appl. Catal. A Gen. 2021, 617. [Google Scholar] [CrossRef]
- Catrinck, M.N.; Barbosa, P.S.; Filho, H.R.O.; Monteiro, R.S.; Barbosa, M.H.P.; Ribas, R.M.; Teófilo, R.F. One-step process to produce furfural from sugarcane bagasse over niobium-based solid acid catalysts in a water medium. Fuel Process. Technol. 2020, 207. [Google Scholar] [CrossRef]
- Yang, F.; Tang, J.; Ou, R.; Guo, Z.; Gao, S.; Wang, Y.; Wang, X.; Chen, L.; Yuan, A. Fully catalytic upgrading synthesis of 5-Ethoxymethylfurfural from biomass-derived 5-Hydroxymethylfurfural over recyclable layered-niobium-molybdate solid acid. Appl. Catal. B Environ. 2019, 256, 117786. [Google Scholar] [CrossRef]
- Campisi, S.; Bennici, S.; Auroux, A.; Carniti, P.; Gervasini, A. A Rational Revisiting of Niobium Oxophosphate Catalysts for Carbohydrate Biomass Reactions. Top. Catal. 2018, 61, 1939–1948. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.J.C.; Granados, M.L.; Gervasini, A.; Carniti, P. Exploitment of niobium oxide effective acidity for xylose dehydration to furfural. Catal. Today 2015, 254, 90–98. [Google Scholar] [CrossRef]
- García-Sancho, C.; Rubio-Caballero, J.M.; Mérida-Robles, J.M.; Moreno-Tost, R.; Santamaría-González, J.; Maireles-Torres, P. Mesoporous Nb2O5 as solid acid catalyst for dehydration of d-xylose into furfural. Catal. Today 2014, 234, 119–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ren, J.; Liu, X.; Li, X.; Xia, Y.; Lu, G.; Wang, Y. Mesoporous niobium phosphate: An excellent solid acid for the dehydration of fructose to 5-hydroxymethylfurfural in water. Catal. Sci. Technol. 2012, 2, 2485–2491. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Biella, S.; Auroux, A. Niobic acid and niobium phosphate as highly acidic viable catalysts in aqueous medium: Fructose dehydration reaction. Catal. Today 2006, 118, 373–378. [Google Scholar] [CrossRef]
- Carlini, C.; Giuttari, M.; Galletti, A.M.R.; Sbrana, G.; Armaroli, T.; Busca, G. Selective saccharides dehydration to 5-hydroxymethyl-2-furaldehyde by heterogeneous niobium catalysts. Appl. Catal. A Gen. 1999, 183, 295–302. [Google Scholar] [CrossRef]
- Kang, S.; Miao, R.; Guo, J.; Fu, J. Sustainable production of fuels and chemicals from biomass over niobium based catalysts: A review. Catal. Today 2020, 374, 61–76. [Google Scholar] [CrossRef]
- Gervasini, A.; Campisi, S.; Carniti, P.; Fantauzzi, M.; Imparato, C.; Clayden, N.J.; Aronne, A.; Rossi, A. Influence of the Nb/P ratio of acidic Nb P Si oxides on surface and catalytic properties. Appl. Catal. A Gen. 2019, 579, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Busca, G. The surface acidity of solid oxides and its characterization by IR spectroscopic methods. An attempt at systematization. Phys. Chem. Chem. Phys. 1999, 1, 723–736. [Google Scholar] [CrossRef]
- Xue, F.; Ma, D.; Tong, T.; Liu, X.; Hu, Y.; Guo, Y.; Wang, Y. Contribution of Different NbOx Species in the Hydrodeoxygenation of 2,5-Dimethyltetrahydrofuran to Hexane. ACS Sustain. Chem. Eng. 2018, 6, 13107–13113. [Google Scholar] [CrossRef]
- Vieira, J.L.; Almeida-Trapp, M.; Mithöfer, A.; Plass, W.; Gallo, J.M.R. Rationalizing the conversion of glucose and xylose catalyzed by a combination of Lewis and Brønsted acids. Catal. Today 2020, 344, 92–101. [Google Scholar] [CrossRef]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Brønsted Acid Sites in the Dehydration of Glycerol over Niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Kim, K.D.; Wang, Z.; Jiang, Y.; Hunger, M.; Huang, J. The cooperative effect of Lewis and Brønsted acid sites on Sn-MCM-41 catalysts for the conversion of 1,3-dihydroxyacetone to ethyl lactate. Green Chem. 2019, 21, 3383–3393. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Bossola, F.; Dal Santo, V. Cooperative action of Brønsted and Lewis acid sites of niobium phosphate catalysts for cellobiose conversion in water. Appl. Catal. B Environ. 2016, 193, 93–102. [Google Scholar] [CrossRef]
- Zaccheria, F.; Santoro, F.; Iftitah, E.D.; Ravasio, N. Brønsted and lewis solid acid catalysts in the valorization of citronellal. Catalysts 2018, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Weingarten, R.; Tompsett, G.A.; Conner, W.C.; Huber, G.W. Design of solid acid catalysts for aqueous-phase dehydration of carbohydrates: The role of Lewis and Brønsted acid sites. J. Catal. 2011, 279, 174–182. [Google Scholar] [CrossRef]
- Santos, K.M.A.; Albuquerque, E.M.; Innocenti, G.; Borges, L.E.P.; Sievers, C.; Fraga, M.A. The Role of Brønsted and Water-Tolerant Lewis Acid Sites in the Cascade Aqueous-Phase Reaction of Triose to Lactic Acid. ChemCatChem 2019, 11, 3054–3063. [Google Scholar] [CrossRef]
- Védrine, J.C. Acid-base characterization of heterogeneous catalysts: An up-to-date overview. Res. Chem. Intermed. 2015, 41, 9387–9423. [Google Scholar] [CrossRef]
- Rocha, A.S.; Costa, G.C.; de Araujo, L.R.R.; Misael, W.A.; Oliveira, R.R.; Rocha, A.B. Insights into the Phosphate Species on Niobia Treated with H3PO4. Catal. Lett. 2019, 150, 1496–1504. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Portilho, M.F.; Silva, A.C.; Taroco, H.A.; Souza, P.P. Modified niobia as a bifunctional catalyst for simultaneous dehydration and oxidation of glycerol. Appl. Catal. B Environ. 2012, 117, 29–35. [Google Scholar] [CrossRef]
- Celdeira, P.A.; Gonçalves, M.; Figueiredo, F.C.A.; Bosco, S.M.D.; Mandelli, D.; Carvalho, W.A. Sulfonated niobia and pillared clay as catalysts in etherification reaction of glycerol. Appl. Catal. A Gen. 2014, 478, 98–106. [Google Scholar] [CrossRef]
- Delgado, D.; Fernández-Arroyo, A.; Domine, M.E.; García-González, E.; López Nieto, J.M. W-Nb-O oxides with tunable acid properties as efficient catalysts for the transformation of biomass-derived oxygenates in aqueous systems. Catal. Sci. Technol. 2019, 9, 3126–3136. [Google Scholar] [CrossRef]
- Kim, M.; Ronchetti, S.; Onida, B.; Ichikuni, N.; Fukuoka, A.; Kato, H.; Nakajima, K. Lewis Acid and Base Catalysis of YNbO4 Toward Aqueous-Phase Conversion of Hexose and Triose Sugars to Lactic Acid in Water. ChemCatChem 2020, 12, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Yang, H.; Wu, S. Efficient conversion of carbohydrates into levulinic acid over chromium modified niobium phosphate catalyst. Fuel 2019, 256, 115940. [Google Scholar] [CrossRef]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Nakajima, K.; Hirata, J.; Kim, M.; Gupta, N.K.; Murayama, T.; Yoshida, A.; Hiyoshi, N.; Fukuoka, A.; Ueda, W. Facile Formation of Lactic Acid from a Triose Sugar in Water over Niobium Oxide with a Deformed Orthorhombic Phase. ACS Catal. 2018, 8, 283–290. [Google Scholar] [CrossRef]
- Somma, F.; Puppinato, A.; Strukul, G. Niobia-silica aerogel mixed oxide catalysts: Effects of the niobium content, the calcination temperature and the surface hydrophilicity on the epoxidation of olefins with hydrogen peroxide. Appl. Catal. A Gen. 2006, 309, 115–121. [Google Scholar] [CrossRef]
- Abdel-Rehim, M.A.; dos Santos, A.C.B.; Camorim, V.L.L.; da Costa Faro, A. Acid-base reactions on alumina-supported niobia. Appl. Catal. A Gen. 2006, 305, 211–218. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Marzo, M. Silica-niobia oxides as viable acid catalysts in water: Effective vs. intrinsic acidity. Catal. Today 2010, 152, 42–47. [Google Scholar] [CrossRef]
- Stošić, D.; Bennici, S.; Rakić, V.; Auroux, A. CeO2–Nb2O5 mixed oxide catalysts: Preparation, characterization and catalytic activity in fructose dehydration reaction. Catal. Today 2012, 192, 160–168. [Google Scholar] [CrossRef]
- Aronne, A.; Di Serio, M.; Vitiello, R.; Clayden, N.J.; Minieri, L.; Imparato, C.; Piccolo, A.; Pernice, P.; Carniti, P.; Gervasini, A. An Environmentally Friendly Nb–P–Si Solid Catalyst for Acid-Demanding Reactions. J. Phys. Chem. C 2017, 121, 17378–17389. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Huang, C.; Sun, X.; Chen, B. Highly active niobium-loaded montmorillonite catalysts for the production of 5-hydroxymethylfurfural from glucose. Green Chem. 2019, 21, 3930–3939. [Google Scholar] [CrossRef]
- Stošić, D.; Bennici, S.; Pavlović, V.; Rakić, V.; Auroux, A. Tuning the acidity of niobia: Characterization and catalytic activity of Nb2O5–MeO2 (Me = Ti, Zr, Ce) mesoporous mixed oxides. Mater. Chem. Phys. 2014, 146, 337–345. [Google Scholar] [CrossRef]
- Fovanna, T.; Campisi, S.; Villa, A.; Kambolis, A.; Peng, G.; Rentsch, D.; Kröcher, O.; Nachtegaal, M.; Ferri, D. Ruthenium on phosphorous-modified alumina as an effective and stable catalyst for catalytic transfer hydrogenation of furfural. RSC Adv. 2020, 10, 11507–11516. [Google Scholar] [CrossRef]
- Campisi, S.; Sanchez Trujillo, F.; Motta, D.; Davies, T.; Dimitratos, N.; Villa, A. Controlling the Incorporation of Phosphorus Functionalities on Carbon Nanofibers: Effects on the Catalytic Performance of Fructose Dehydration. C 2018, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Campisi, S.; Capelli, S.; Motta, D.; Trujillo, F.; Davies, T.; Prati, L.; Dimitratos, N.; Villa, A. Catalytic Performances of Au–Pt Nanoparticles on Phosphorous Functionalized Carbon Nanofibers towards HMF Oxidation. C 2018, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Shivhare, A.; Kumar, A.; Srivastava, R. Metal phosphate catalysts to upgrade lignocellulose biomass into value-added chemicals and biofuels. Green Chem. 2021, 23, 3818–3841. [Google Scholar] [CrossRef]
- Catrinck, M.N.; Ribeiro, E.S.; Monteiro, R.S.; Ribas, R.M.; Barbosa, M.H.P.; Teófilo, R.F. Direct conversion of glucose to 5-hydroxymethylfurfural using a mixture of niobic acid and niobium phosphate as a solid acid catalyst. Fuel 2017, 210, 67–74. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Berry, F.J. The oxidation states of titanium and niobium in compounds of composition SnxNbTiP3O12 (0 <x ≤ 0.50): An XPS study. Polyhedron 1997, 16, 2957–2961. [Google Scholar] [CrossRef]
- Francisco, M.S.P.; Landers, R.; Gushikem, Y. Local order structure and surface acidity properties of a Nb2O5/SiO2 mixed oxide prepared by the sol-gel processing method. J. Solid State Chem. 2004, 177, 2432–2439. [Google Scholar] [CrossRef]
- Okuhara, T. Water-tolerant solid acid catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; Van Der Voort Maarschalk, K.; Hinrichs, W.L.J. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, J.; Li, X.; Liu, X.; Xia, Y.; Hu, B.; Lu, G.; Wang, Y. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurural over water-tolerant niobium-based catalysts. Fuel 2015, 139, 301–307. [Google Scholar] [CrossRef]
- Apolinário, A.C.; De Lima Damasceno, B.P.G.; De Macêdo Beltrão, N.E.; Pessoa, A.; Converti, A.; Da Silva, J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef]
- Singh, R.S.; Chauhan, K.; Pandey, A.; Larroche, C. Biocatalytic strategies for the production of high fructose syrup from inulin. Bioresour. Technol. 2018, 260, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.S.; Li, X.L.; Xu, H.J.; Fu, Y. Conversion of 5-hydroxymethylfurfural to chemicals: A review of catalytic routes and product applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Van Der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. The effect of solvent addition on fructose dehydration to 5-hydroxymethylfurfural in biphasic system over zeolites. J. Catal. 2012, 287, 68–75. [Google Scholar] [CrossRef]
- Fu, X.; Hu, Y.; Zhang, Y.; Zhang, Y.; Tang, D.; Zhu, L.; Hu, C. Solvent Effects on Degradative Condensation Side Reactions of Fructose in Its Initial Conversion to 5-Hydroxymethylfurfural. ChemSusChem 2020, 13, 438. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Marzo, M. Absence of expected side-reactions in the dehydration reaction of fructose to HMF in water over niobic acid catalyst. Catal. Commun. 2011, 12, 1122–1126. [Google Scholar] [CrossRef]
- Ingle, J.D.; Crouch, S.R. Simultaneous determination of silicate and phosphate by an automated differential kinetic procedure. Anal. Chem. 1971, 43, 7–10. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Ferri, M.; Campisi, S.; Scavini, M.; Evangelisti, C.; Carniti, P.; Gervasini, A. In-depth study of the mechanism of heavy metal trapping on the surface of hydroxyapatite. Appl. Surf. Sci. 2019, 475, 397–409. [Google Scholar] [CrossRef]
- Schieppati, D.; Patience, N.A.; Campisi, S.; Patience, G.S. Experimental methods in chemical engineering: High performance liquid chromatography—HPLC. Can. J. Chem. Eng. 2021, 99, 1663–1682. [Google Scholar] [CrossRef]









| Code | Nominal Composition (wt%) | Normalized P Content | |||
|---|---|---|---|---|---|
| Nb | P | O | K a | ||
| NbO | 69.9 | - | 30.1 | - | 0 |
| NbO:NbP(3:1) | 66.0 | 2.1 | 31.3 | 0.5 | 0.25 |
| NbO:NbP(1:1) | 62.3 | 4.2 | 32.5 | 1.1 | 0.5 |
| NbO:NbP(1:3) | 58.6 | 6.2 | 33.6 | 1.6 | 0.75 |
| NbP | 55.0 | 8.2 | 34.7 | 2.1 | 1 |
| Catalyst | Inulin Hydrolysis a | ||||
|---|---|---|---|---|---|
| kT b (h−1) | Ea (kJ mol−1) | lnA (A in h−1) | |||
| 70 °C | 80 °C | 90 °C | |||
| NbO | 0.00304 | 0.00457 | 0.00387 | n.d b | n.d. b |
| NbO:NbP(3:1) | 0.00304 | 0.00771 | 0.0763 | 107.1 ± 20.1 | 32.6 ± 6.9 |
| NbO:NbP(1:1) | 0.00721 | 0.0625 | 0.142 | 99.8 ± 8.8 | 31.1 ± 3.0 |
| NbO:NbP(1:3) | 0.0312 | 0.0808 | 0.307 | 123.9 ± 6.5 | 40.1 ± 2.2 |
| NbP | 0.0373 | 0.141 | 0.571 | 136 ± 5.3 | 44.6 ± 1.8 |
| Samples | Initial Velocity a(h−1) | k120 b (min−1∙mL gcat) | |
|---|---|---|---|
| τ = 6 min mL−1 gcat −1 | τ = 15 min mL−1 gcat −1 | ||
| NbO | 0.27 | 0.10 | 0.011 |
| NbO:NbP(3:1) | 0.31 | 0.13 | 0.017 |
| NbO:NbP(1:1) | 0.38 | 0.20 | 0.021 |
| NbP | 0.36 | 0.20 | 0.061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catrinck, M.N.; Campisi, S.; Carniti, P.; Teófilo, R.F.; Bossola, F.; Gervasini, A. Phosphate Enrichment of Niobium-Based Catalytic Surfaces in Relation to Reactions of Carbohydrate Biomass Conversion: The Case Studies of Inulin Hydrolysis and Fructose Dehydration. Catalysts 2021, 11, 1077. https://doi.org/10.3390/catal11091077
Catrinck MN, Campisi S, Carniti P, Teófilo RF, Bossola F, Gervasini A. Phosphate Enrichment of Niobium-Based Catalytic Surfaces in Relation to Reactions of Carbohydrate Biomass Conversion: The Case Studies of Inulin Hydrolysis and Fructose Dehydration. Catalysts. 2021; 11(9):1077. https://doi.org/10.3390/catal11091077
Chicago/Turabian StyleCatrinck, Mariana N., Sebastiano Campisi, Paolo Carniti, Reinaldo F. Teófilo, Filippo Bossola, and Antonella Gervasini. 2021. "Phosphate Enrichment of Niobium-Based Catalytic Surfaces in Relation to Reactions of Carbohydrate Biomass Conversion: The Case Studies of Inulin Hydrolysis and Fructose Dehydration" Catalysts 11, no. 9: 1077. https://doi.org/10.3390/catal11091077
APA StyleCatrinck, M. N., Campisi, S., Carniti, P., Teófilo, R. F., Bossola, F., & Gervasini, A. (2021). Phosphate Enrichment of Niobium-Based Catalytic Surfaces in Relation to Reactions of Carbohydrate Biomass Conversion: The Case Studies of Inulin Hydrolysis and Fructose Dehydration. Catalysts, 11(9), 1077. https://doi.org/10.3390/catal11091077