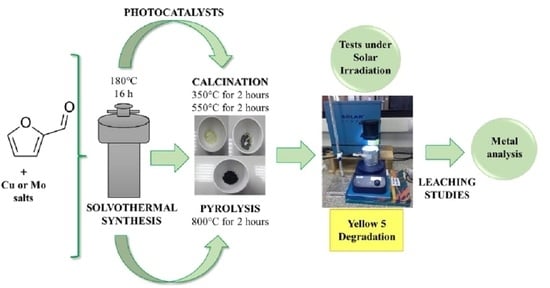
Photocatalytic Performance of Carbon-Containing CuMo-Based Catalysts under Sunlight Illumination
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
2.1.1. Yields and Proximate Analysis
2.1.2. Electron Microscopy and EDS Analysis
2.1.3. Textural Characterization
2.1.4. XRD Patterns
2.1.5. Optical Characterization by Diffuse Reflectance
2.2. Adsorption Studies of Dye Y5
2.3. Photocatalytic Tests on Cu-Based Photocatalysts
2.4. Leaching Studies of the Mo-Based Photocatalysts
2.5. General Discussion
3. Experimental
3.1. Materials and Synthesis of Photocatalysts
3.2. Characterization of Materials
3.3. Adsorption and Photocatalytic Tests
3.4. Lixiviation Tests
3.5. Scavenger Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Declaration
References
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption Characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSA Adv. 2014, 4, 37003–37026. [Google Scholar]
- Greluk, M.; Hubicki, Z. Efficient removal of acide orange 7 dye from water using the strongly basic anion exchange resin Amberlite IRA-95. Desalination 2011, 278, 219–226. [Google Scholar] [CrossRef]
- Malik, A.; Grohmann, E. Environmental Protection Strategies for Sustainable Development; Springer: New York, NY, USA, 2012. [Google Scholar]
- He, X.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assesments. J. Food. Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. FDA. Listing of color additives exempt from certification. In Code of Federal Regulations Title 21–Food and Drugs; Office USGP, Ed.; FDA: Washington, DC, USA, 2002. [Google Scholar]
- Maekawa, A.; Matsuoka, C.; Onodera, H.; Tanigawa, H.; Furuta, K.; Kanno, J.; Jang, J.J.; Hayashi, Y.; Ogiu, T. Lack of carcinogenicity of tartrazine (FD & C Yellow No. 5) in the F344 rat. Food Chem. Toxicol. 1987, 25, 891–896. [Google Scholar] [PubMed]
- Kapadia, G.J.; Tokuda, H.; Sridhar, R.; Balasubramanian, V.; Takayasu, J.; Bu, P.; Enjo, F.; Takasaki, M.; Konoshima, T.; Nishino, H. Cancer chemopreventive activity of synthetic colorants used in foods, pharmaceuticals and cosmetic preparations. Cancer Lett. 1998, 129, 87–95. [Google Scholar] [CrossRef]
- Turner, P.J.; Kemp, A.S. Intolerance to food additives–does it exists? J. Paediatr. Child Health 2012, 48, E10–E14. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behavior in 3-year-old and 8/9-year-old children in the community: A randomized, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef] [Green Version]
- Oppenlander, T. Photochemical Purification of Water and Air: Advanced Oxidation Processes (AOPs): Principles, Reaction Mechanisms, Reactor Concepts; Wiley-VHC: Hoboken, NJ, USA, 2003. [Google Scholar]
- Bhat, A.P.; Gogate, P.R. Degradation of nitrogen-containing hazardous compounds using advanced oxidation processes: A review on aliphatic and aromatic amines, dyes, and pesticides. J. Hazard. Mater. 2021, 403, 123657. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirnioti, P.G. Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl. Catal. B Environ. 2014, 144, 333–342. [Google Scholar] [CrossRef]
- Miljević, B.; Van der Bergh, J.M.; Vučetić, S.; Lazar, D.; Ranogajec, J. Molybdenum doped TiO2 nanocomposite coatings: Visible light driven photocatalytic self-cleaning of mineral substrates. Ceram. Int. 2017, 43, 8214–8221. [Google Scholar] [CrossRef]
- Kozlov, Y.A.; Dorogov, M.V.; Chirkunova, N.V.; Sosnin, I.M.; Vikarchuk, A.A.; Romanov, A.E. CuO Nanowhiskers-based photocatalysts for wastewater treatment. Nano Hybrids Compos. 2017, 13, 183–189. [Google Scholar] [CrossRef]
- An, X.; Liu, H.; Qu, J.; Moniza, S.J.A.; Tang, J. Photocatalytic mineralization of herbicide 2,4,5-trichlorophenoxyacetic acid: Enhanced performance by triple junction Cu–TiO2–Cu2O and the underlying reaction mechanism. New J. Chem. 2015, 39, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Andrade, M.A.; Carmona, R.J.; Mestre, A.S.; Matos, J.; Carvalho, A.P.; Ania, C.O. Visible light driven photooxidation of phenol on TiO2/Cu-loaded carbon catalysts. Carbon 2014, 76, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Leen, S.C.; Hasan, N.; Lintang, H.O.; Shamsuddin, M.; Leny, Y. Photocatalytic removal of 2,4-dichlorophenoxyacetic acid herbicide on copper oxide/titanium dioxide prepared by co-precipitation method. Mater. Sci. Eng. 2016, 107, 012012. [Google Scholar]
- Alam, U.; Kumar, S.; Bahnemann, D.; Koch, J.; Tegenkamp, C.; Muneer, M. Harvesting visible light with MoO3 nanorods photocatalytic degradation of organic pollutants. Phys. Chem. Chem. Phys. 2018, 20, 4538–4545. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fang, W.; Yang, Y.; Wu, J.; Yu, H.; Dong, X.; Wang, T.; Liu, Z.; Zhao, B. Three-dimensional MoO3 nanoflowers assembled with nanosheets for rhodamine B degradation under visible light. Mater. Res. Bull. 2018, 108, 38–45. [Google Scholar] [CrossRef]
- Kumar, V.V.; Gayathri, K.; Anthony, S.P. Synthesis of α-MoO3 nanoplates using organic aliphatic acids and investigation of sunlight enhanced photodegradation of organic dyes. Mater. Res. Bull. 2016, 76, 147–154. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Sanjini, N.S.; Chandra Bose, A.; Velmathi, S. Flower-like hierarchical h-MoO3: New findings of efficient visible light driven nano photocatalyst for methylene blue degradation. Catal. Sci. Technol. 2013, 3, 1405–1414. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Velasco, L.F.; Velo-Gala, I.; Ania, C.O. Photochemistry based on nanoporous carbons: Perspectives in energy conversion and environmental remediation. J. Colloid Interf. Sci. 2017, 490, 879–901. [Google Scholar] [CrossRef] [Green Version]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 39, 741–772. [Google Scholar] [CrossRef]
- Gao, R.; Folens, K.; Mees, B.; Laing, G.D.; Rabaey, K.; Bonin, L. Copper and zinc extraction from automobile shredder residues via an integrated electrodeposition and crystallization process. Resour. Conserv. Recycl. 2021, 172, 105672. [Google Scholar] [CrossRef]
- Pedroza-Herrera, G.; Medina-Ramírez, I.E.; Lozano-Álvarez, J.A.; Rodil, S.E. Evaluation of the photocatalytic activity of copper doped TiO2 nanoparticles for the purification and/or disinfection of industrial effluents. Catal. Today 2020, 341, 37–48. [Google Scholar] [CrossRef]
- Díaz-Martínez, M.E.; Argumedo-Delira, R.; Sánchez-Viveros, G.; Alarcón, A.; Mendoza-López, M.R. Microbial bioleaching of Ag, Au and Cu from printed circuit boards of mobile phones. Curr. Microbiol. 2019, 76, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Reyes, M.; Camposeco-Solís, R.; Zanella, R.; Rodríguez-González, V. Hydrogen production by tailoring the brookite and Cu2O ratio of sol-gel Cu-TiO2 photocatalysts. Chemosphere 2017, 184, 992–1002. [Google Scholar] [CrossRef]
- Mitchell, P.C.H. Speciation of Molybdenum Compounds in Water. Ultraviolet Spectra and REACH Read across Report for the International Molybdenum Association; REACH Molybdenum Consortium: London, UK, 2009; pp. 1–28. [Google Scholar]
- Gopalakrishnana, R.; Ashokkumar, M. Rare earth metals (Ce and Nd) induced modifications on structural, morphological, and photoluminescence properties of CuO nanoparticles and antibacterial application. J. Mol. Struct. 2021, 1244, 131207. [Google Scholar] [CrossRef]
- Varakin, A.N.; Mozhaev, A.V.; Pimerzin, A.A.; Nikulshin, P.A. Toward HYD/DEC selectivity control in hydrodeoxygenation over supported and unsupported Co(Ni)-MoS2 catalysts. A key to effective dual-bed catalyst reactor for co-hydroprocessing of diesel and vegetable oil. Catal. Today 2020, 357, 556–564. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, C.; Sun, W.; Zhu, G.; Du, A.; Zhang, H. In-situ conversion growth of carbon-coated MoS2/N-doped carbon nanotubes as anodes with superior capacity retention for sodium-ion batteries. J. Mater. Sci. Technol. 2022, 102, 8–15. [Google Scholar] [CrossRef]
- Matos, J.; Ocares-Riquelme, J.; Poon, P.S.; Montaña, R.; García, X.; Campos, K.; Hernández-Garrido, J.C.; Titirici, M.M. C-doped anatase TiO2: Adsorption kinetics and photocatalytic degradation of methylene blue and phenol, and correlations with DFT estimations. J. Colloid Interface Sci. 2019, 547, 14–29. [Google Scholar] [CrossRef]
- Ito, T.; Takagi, H.; Asano, T. Drastic and sharp change in color, shape, and magnetism in transition of CuMoO4 single crystals. Chem. Mater. 2009, 21, 3376–3379. [Google Scholar] [CrossRef]
- Du, D.; Lan, R.; Xu, W.; Beanland, R.; Wang, H.; Tao, S. Preparation of a hybrid Cu2O/CuMoO4 nanosheet electrode for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 17749–17756. [Google Scholar] [CrossRef] [Green Version]
- Nassau, K.; Shiever, J.W. Cupric oxide-molybdenum oxide phase diagram in air and in oxygen. J. Am. Ceram. Soc. 1969, 52, 36. [Google Scholar] [CrossRef]
- Hamasaki, T.; Ide, T.; Kuroe, H.; Sekine, T.; Hase, M.; Tsukada, I.; Sakakibara, T. Successive phase transitions to antiferromagnetic and weak-ferromagnetic long-range order in the quasi-one-dimensional antiferromagnet Cu3Mo2O9. Phys. Rev. B 2008, 77, 134419. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.; Miranda, C.; Poon, P.S.; Mansilla, H.D. Nanostructured hybrid TiO2-C for the photocatalytic conversion of phenol. Sol. Energy 2016, 134, 64–71. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Arfan, M.; Siddiqui, D.N.; Shahid, T.; Iqbal, Z.; Majeed, Y.; Akram, I.; Noreen; Bagheri, R.; Song, Z.; Zeb, A. Tailoring of nanostructures: Al doped CuO synthesized by composite-hydroxide-mediated approach. Res. Phys. 2019, 13, 1021872. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, C.; Gao, G.; Cui, D. Facile synthesis of hollow Cu2O octahedral and spherical nanocrystals and their morphology-dependent photocatalytic properties. Nanoscale Res. Lett. 2012, 7, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theerthagiri, J.; Senthil, R.A.; Buraidah, M.H.; Madhavan, J.; Arof, A.K. Synthesis of α-Mo2C by carburization of α-MoO3 nanowires and its electrocatalytic activity towards tri-iodide reduction for dye-sensitized solar cells. J. Mater. Sci. Technol. 2016, 32, 1339–1344. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Tian, Y.; Zhao, D.; Hu, C.; Cao, M. A simple reduction process to synthesize MoO2/C composites with cage-like structure for high performance lithium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 8831–8837. [Google Scholar] [CrossRef]
- Tamboli, P.S.; Prasad, M.B.R.; Kadam, V.S.; Vhatkar, R.S.; Inamuddin, P.H.M.; Mahajan, S.S. α-MoO3-C composite as counter electrode for quantum dot sensitized solar cells. Sol. Energy Mater. Sol. Cells 2017, 161, 96–101. [Google Scholar] [CrossRef]
- Jin, Q.; Fujishima, M.; Iwaszuk, A.; Nolan, M.; Tada, H. Loading effect in copper (II) oxide cluster-surface-modified titanium (IV) oxide on visible-and UV-light activities. J. Phys. Chem. C 2013, 117, 23848–23857. [Google Scholar] [CrossRef] [Green Version]
- Sohrabnezhada, S.; Valipour, A. Synthesis of Cu/CuO nanoparticles in mesoporous material by solid state reaction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 298–302. [Google Scholar] [CrossRef]
- Kang, Z.; Yan, X.; Wang, Y.; Bai, Z.; Liu, Y.; Zhang, Z.; Lin, P.; Zhang, X.; Yuan, H.; Zhang, X.; et al. Electronic Structure Engineering of Cu2O Film/ZnO Nanorods Array All-Oxide p-n Heterostructure for Enhanced Photoelectrochemical Property and Self-powered Biosensing Application. Sci. Rep. 2015, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, S.; Hassanzadeh-Tabrizi, S.A. MoO3 fibers and belts: Molten salt synthesis, characterization and optical properties. Ceram. Int. 2015, 4, 10839–10843. [Google Scholar] [CrossRef]
- Szkoda, M.; Trzcinski, K.; Nowak, A.P.; Gazda, M.; Sawczak, M.; Lisowska-Oleksiak, A. The effect of morphology and crystalline structure of Mo/MoO3 layers on photocatalytic degradation of water organic pollutants. Mater. Chem. Phys. 2020, 248, 122908. [Google Scholar] [CrossRef]
- Chen, G.; Nengzi, L.C.; Gao, Y.; Zhu, G.; Gou, J.; Cheng, X. Degradation of tartrazine by peroxymonosulfate through magnetic Fe2O3/Mn2O3 composites activation. Chin. Chem. Lett. 2020, 31, 2730–2736. [Google Scholar] [CrossRef]
- Souza, I.P.A.F.; Pezoti, O.; Bedin, K.C.; Cazetta, A.L.; Melo, S.A.R.; Souza, L.S.; Silva, M.C.; Almeida, V.C. Chemometric study of thermal treatment effect on the P25 photoactivity for degradation of tartrazine yellow dye. Ceram. Int. 2018, 44, 12292–12300. [Google Scholar] [CrossRef]
- Mann, R.S.; Khulbe, K.C. ESR study of MoO3 obtained from thermal decomposition of ammonium molybdate. Bull. Chem. Soc. Jpn. 1975, 48, 1021–1023. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Jain, R.; Nayak, A.; Agarwal, S.; Shrivastava, M. Removal of the hazardous dye-Tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. 2011, 31, 1062–1067. [Google Scholar] [CrossRef]
- Ramasamy Raja, V.; Rani Rosaline, D.; Suganthia, A.; Rajarajan, M. Facile sonochemical synthesis of Zn2SnO4-V2O5 nanocomposite as an effective photocatalyst for degradation of Eosin Yellow. Ultrason. Sonochemistry 2018, 44, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhao, Z.J.; Zhang, G.; Yang, P.; Li, L.; Gao, H.; Liu, S.; Chang, X.; Chen, S.; Wang, T.; et al. The nature of active sites for carbon dioxide electroreduction over oxide-derived copper catalysts. Nat. Commun. 2021, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Saji, V.S.; Lee, C.W. Molybdenum, molybdenum oxides, and their electrochemistry. ChemSusChem 2021, 5, 1146–1161. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J. Ethylene conversion on activated carbon supported NiMo catalysts: Effect of the support. Appl. Catal. A Gen. 2003, 241, 25–38. [Google Scholar] [CrossRef]
- Goscianska, J.; Pietzrak, R.; Matos, J. Catalytic performance of ordered mesoporous carbons modified with lanthanides in dry methane reforming. Catal. Today 2018, 301, 204–216. [Google Scholar] [CrossRef]
- Popadić, M.G.; Marinović, S.R.; Mudrinić, T.M.; Milutinović-Nikolić, A.D.; Banković, P.T.; Đorđević, I.S.; Janjić, G.V. A novel approach in revealing mechanisms and particular step predictors of pH dependent tartrazine catalytic degradation in presence of Oxone®. Chemosphere 2021, 281, 130806. [Google Scholar] [CrossRef]
- Ali, L.; Algaithi, R.; Habib, H.M.; Souka, U.; Rauf, M.A.; Ashraf, S.S. Soybean peroxidase-mediated degradation of an azo dye–A detailed mechanistic study. BMC Biochem. 2013, 14, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza-Huizar, L.H. A theoretical study of chemical reactivity of tartrazine through DFT reactivity descriptors. J. Mex. Chem. Soc. 2014, 58, 416–423. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Inagaki, M.; Nonaka, M.; Kojin, F.; Tsumura, T.; Toyoda, M. Cyclic performance of carbon-coated TiO2 for photocatalytic activity of methylene blue decomposition. Environ. Technol. 2006, 27, 521–528. [Google Scholar] [CrossRef]
- Carvalho, H.W.P.; Batista, A.P.L.; Hammer, P.; Ramalho, T.C. Photocatalytic degradation of methylene blue by TiO2-Cu thin films: Theoretical and experimental study. J. Hazard. Mater. 2010, 184, 273–280. [Google Scholar] [CrossRef]
- Acosta-Silva, Y.J.; Nava, R.; Hernández-Morales, V.; Macías-Sánchez, S.A.; Gómez-Herrera, M.L.; Pawelec, B. Methylene blue photodegradation over titania-decorated SBA-15. Appl. Catal. B Environ. 2011, 110, 108–117. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sadaba, I.; López-Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Swanson, H.E.; Fuyat, R.K. Standard X-ray Diffraction Powder Patterns. National Bureau of Standards Circular 539 Volume II. 1954. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/MONO/nbsmonograph25-5.pdf (accessed on 26 November 2021).
- Swanson, H.E.; Fuyat, R.K.; Ugrinic, G.M. Standard X-ray Diffraction Powder Patterns. National Bureau of Standards Circular 539 Volume III. 1954. Available online: https://www.govinfo.gov/content/pkg/GOVPUB-C13-16bbe9001e466cca001840ef229edb36/pdf/GOVPUB-C13-16bbe9001e466cca001840ef229edb36.pdf (accessed on 26 November 2021).
- Brandani, S. Kinetics of liquid phase batch adsorption experiments. Adsorption 2021, 27, 353–368. [Google Scholar] [CrossRef]












| Sample | C (wt.%) a | O (wt.%) a | Cu (wt.%) a | Mo (wt.%) a | C (wt.%) b | O (wt.%) b | Cu (wt.%) b | Mo (wt.%) b | Other (wt.%) b |
|---|---|---|---|---|---|---|---|---|---|
| Cu4 | 83.36 | 13.79 | 2.85 | - | 41.01 | 5.29 | 53.70 | - | - |
| Cu4-350-O2 | 72.52 | 20.35 | 7.13 | - | 17.43 | 7.51 | 75.05 | - | - |
| Cu4-550-O2 | 61.31 | 23.66 | 15.04 | - | 10.28 | 13.08 | 76.64 | - | - |
| Cu4-800-N2 | 87.87 | 4.69 | 7.44 | - | 25.17 | 3.19 | 71.64 | - | - |
| Mo4 | 76.66 | 22.20 | - | 1.14 | 27.99 | 24.63 | - | 42.58 | 4.80 c |
| Mo4-350-O2 | 28.09 | 31.26 | - | 40.65 | 17.24 | 28.24 | - | 54.52 | - |
| Mo4-550-O2 | 14.17 d | 17.74 d | - | 68.10 d | 11.41 | 27.92 | - | 60.67 | - |
| Mo4-800-N2 | 41.79 | 16.45 | - | 41.77 | 10.56 | 20.13 | - | 69.31 | - |
| Cu4Mo4 | 61.14 | 22.04 | 9.32 | 7.50 | 61.00 | 15.30 | 16.50 | 7.21 | - |
| Cu4Mo4-350-O2 | 48.24 | 22.56 | 9.40 | 19.80 | 37.85 | 24.71 | 11.34 | 20.05 | - |
| Cu4Mo4-550-O2 | 36.56 d | 27.10 d | 12.12 d | 27.22 d | 32.07 | 30.49 | 17.12 | 20.32 | - |
| Cu4Mo4-800-N2 | 66.58 | 10.96 | 20.63 | 1.83 | 33.85 | 6.05 | 50.44 | 9.65 | - |
| Sample | SBET (m2/g) a | Vtotal (cm3/g) b | Vmicro (cm3/g) c | Fraction of Micropores (%) d |
|---|---|---|---|---|
| Cu4 | 15 | 0.029 | 0.003 | 10 |
| Cu4-350-O2 | 4 | 0.010 | 0.001 | 10 |
| Cu4-550-O2 | 6 | 0.007 | 0.001 | 14 |
| Cu4-800-N2 | 90 | 0.073 | 0.035 | 48 |
| Mo4 | 1 | 0.003 | <0.001 | 0 |
| Mo4-350-O2 | 10 | 0.065 | 0.003 | 5 |
| Mo4-550-O2 | 7 | 0.006 | 0.001 | 17 |
| Mo4-800-N2 | 18 | 0.039 | 0.010 | 26 |
| Cu4Mo4 | 6 | 0.044 | 0.001 | 2 |
| Cu4Mo4-350-O2 | 6 | 0.027 | 0.002 | 7 |
| Cu4Mo4-550-O2 | 4 | 0.004 | <0.001 | 0 |
| Cu4Mo4-800-N2 | 14 | 0.050 | 0.004 | 8 |
| Sample | Crystalline Phase a | Index Numbers b | 2θ c | FWHM (rad) d | D (nm) e | JCPDS Card f |
|---|---|---|---|---|---|---|
| Cu4 | Cu | (111) | 43.26 | 0.0045 | 30 | 01-085-1326 |
| Cu4-350-O2 | CuO | (111) | 38.96 | 0.0068 | 20 | 01-072-0629 |
| Cu4-550-O2 | CuO | (-111) | 35.56 | 0.0043 | 32 | 01-072-0629 |
| Cu4-800-N2 | Cu | (111) | 43.29 | 0.0036 | 38 | 01-085-1326 |
| CuO | (111) | 38.69 | 0.0070 | 19 | 01-072-0629 | |
| Cu2O | (111) | 36.42 | 0.0059 | 23 | 05-0667 | |
| Mo4 | MoO3 | (021) | 25.82 | 0.0033 | 43 | 05-0508 |
| Mo4-350-O2 | MoO3 | (021) | 27.31 | 0.0032 | 45 | 05-0508 |
| Mo4-550-O2 | MoO3 | (040) | 25.75 | 0.0019 | 74 | 05-0508 |
| Mo4-800-N2 | MoO3 | (040) | 25.92 | 0.0029 | 48 | 05-0508 |
| Cu4Mo4 | Cu | (111) | 43.33 | 0.0041 | 33 | 01-085-1326 |
| Cu4Mo4-350-O2 | MoO3 | (021) | 27.29 | 0.0030 | 47 | 05-0508 |
| Cu4Mo4-550-O2 | MoO3 | (040) | 25.69 | 0.0023 | 63 | 05-0508 |
| Cu2O | (111) | 29.34 | 0.0026 | 53 | 05-0667 | |
| Cu4Mo4-800-N2 | Cu | (120) | 43.35 | 0.0034 | 39 | 01-085-1326 |
| MoO3 | (111) | 26.04 | 0.0032 | 44 | 032-0671 |
| Sample | nads a (μmol) | k1 (min−1) b | Rk1 c | k2 (μmol−1 min−1) d | Rk2 e | kp (μmol min−1/2) f | Rkp g | C (μmol) h |
|---|---|---|---|---|---|---|---|---|
| Cu4 | 0.034 | 0.097 | 0.945 | 9.80 | 0.973 | 0.002 | 0.918 | 0.021 |
| Cu4-350-O2 | 0.044 | 0.035 | 0.886 | 1.62 | 0.973 | 0.005 | 0.984 | 0.007 |
| Cu4-550-O2 | 0.075 | 0.037 | 0.969 | 0.99 | 0.975 | 0.009 | 0.991 | 0.002 |
| Cu4-800-N2 | 0.250 | 0.033 | 0.941 | 0.25 | 0.984 | 0.030 | 0.982 | 0.011 |
| Mo4 | 0.044 | 0.080 | 0.929 | 1.34 | 0.930 | 0.006 | 0.896 | 0.003 |
| Mo4-350-O2 | 0.104 | 0.050 | 0.992 | 1.06 | 0.949 | 0.017 | 0.966 | −0.020 |
| Mo4-550-O2 | 0.030 | 0.025 | 0.993 | 1.28 | 0.999 | 0.005 | 0.973 | −0.007 |
| Mo4-800-N2 | 0.129 | 0.043 | 0.850 | 0.80 | 0.969 | 0.011 | 0.993 | 0.042 |
| Cu4Mo4 | 0.035 | 0.047 | 0.980 | 3.21 | 0.969 | 0.004 | 0.995 | 0.003 |
| Cu4Mo4-350-O2 | 0.062 | 0.031 | 0.994 | 0.84 | 0.997 | 0.009 | 0.994 | −0.010 |
| Cu4Mo4-550-O2 | 0.057 | 0.041 | 0.898 | 1.63 | 0.975 | 0.006 | 0.991 | 0.013 |
| Cu4Mo4-800-N2 | 0.332 | 0.043 | 0.985 | 0.28 | 0.986 | 0.041 | 0.999 | 0.016 |
| Sample | nads a (μmol) | kapp × 10−3 (min−1) b | Rkapp c | ϕrel-DP d (a.u) | ϕrel-Cu4 e (a.u) | Y5conv-6h f (%) |
|---|---|---|---|---|---|---|
| Direct Photolysis (DP) | - | 0.5 | 0.968 | 1.0 | 0.8 | 9 |
| TiO2-P25 | 0.960 | 111 | 0.950 | 222 | 185 | 100 (1 h) |
| Cu4 | 0.034 | 0.6 | 0.943 | 1.2 | 1.0 | 16 |
| Cu4-350-O2 | 0.044 | 0.9 | 0.973 | 1.8 | 1.5 | 19 |
| Cu4-550-O2 | 0.075 | 0.9 | 0.989 | 1.8 | 1.5 | 19 |
| Cu4-800-N2 | 0.250 | 2.6 | 0.948 | 5.2 | 4.3 | 40 |
| Catalysts | Loading of Catalyst (mg L−1) | Nominal Cu (mg L−1) | Cu Leached (mg L−1) | Nominal Mo (mg L−1) | Mo Leached (mg L−1) |
|---|---|---|---|---|---|
| Cu4 | 48.2 | 21.8 a | <5 | 0 | 0 |
| Cu4-350-O2 | 50.3 | 45.8 | <5 | 0 | 0 |
| Cu4-550-O2 | 50.2 | 49.1 | 0 | 0 | 0 |
| Cu4-800-N2 | 53.1 | 41.5 | <5 | 0 | 0 |
| Mo4 | 53.4 | 0 | 0 | 39.6 a | >30 |
| Mo4-350-O2 | 52.4 | 0 | 0 | 49.8 | >50 |
| Mo4-550-O2 | 54.6 | 0 | 0 | 53.9 | >20 |
| Mo4-800-N2 | 51.5 | 0 | 0 | 47.0 | >5 |
| Cu4Mo4 | 51.7 | 16.3 a | <10 | 26.8 | >20 |
| Cu4Mo4-350-O2 | 53.8 | 25.4 | <10 | 26.5 | >20 |
| Cu4Mo4-550-O2 | 51.8 | 25.6 | <5 | 25.8 | >20 |
| Cu4Mo4-800-N2 | 49.6 | 21.8 | <10 | 25.4 | >20 |
| Catalysts | Leached Cu after 1 h in Dark (wt.%) a | Cu Leached after 5 h Irradiation (wt.%) a | Leached Mo after 1 h in Dark (wt.%) a | Leached Mo after 5 h Irradiation (wt.%) a |
|---|---|---|---|---|
| Cu4 | 0.1 | 0.4 | 0 | 0 |
| Cu4-350-O2 | <0.1 | 0.1 | 0 | 0 |
| Cu4-550-O2 | <0.1 | <0.1 | 0 | 0 |
| Cu4-800-N2 | <0.1 | 0.2 | 0 | 0 |
| Mo4 | 0 | 0 | 69 | 95 |
| Mo4-350-O2 | 0 | 0 | 60 | 100 |
| Mo4-550-O2 | 0 | 0 | 15 | 40 |
| Mo4-800-N2 | 0 | 0 | 10 | 28 |
| Cu4Mo4 | 0.1 | 0.5 | 32 | 100 |
| Cu4Mo4-350-O2 | 0.9 | 1.0 | 59 | 100 |
| Cu4Mo4-550-O2 | 0.1 | 0.3 | 39 | 100 |
| Cu4Mo4-800-N2 | 0.1 | 0.3 | 10 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Flores, P.; Poon, P.S.; Sepulveda, C.; Ania, C.O.; Matos, J. Photocatalytic Performance of Carbon-Containing CuMo-Based Catalysts under Sunlight Illumination. Catalysts 2022, 12, 46. https://doi.org/10.3390/catal12010046
Muñoz-Flores P, Poon PS, Sepulveda C, Ania CO, Matos J. Photocatalytic Performance of Carbon-Containing CuMo-Based Catalysts under Sunlight Illumination. Catalysts. 2022; 12(1):46. https://doi.org/10.3390/catal12010046
Chicago/Turabian StyleMuñoz-Flores, Paula, Po S. Poon, Catherine Sepulveda, Conchi O. Ania, and Juan Matos. 2022. "Photocatalytic Performance of Carbon-Containing CuMo-Based Catalysts under Sunlight Illumination" Catalysts 12, no. 1: 46. https://doi.org/10.3390/catal12010046
APA StyleMuñoz-Flores, P., Poon, P. S., Sepulveda, C., Ania, C. O., & Matos, J. (2022). Photocatalytic Performance of Carbon-Containing CuMo-Based Catalysts under Sunlight Illumination. Catalysts, 12(1), 46. https://doi.org/10.3390/catal12010046