Lipases as Effective Green Biocatalysts for Phytosterol Esters’ Production: A Review
Abstract
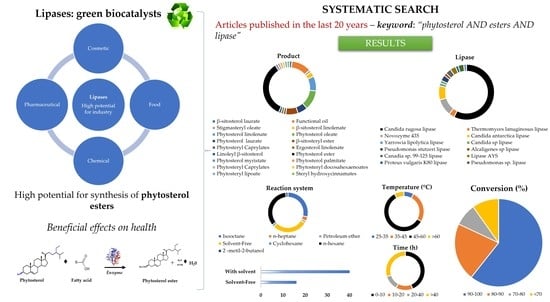
:1. Introduction
2. Lipases
3. Phytosterol Esters
3.1. Roles of Bioactive Compounds
3.2. Phytosterols
3.3. Synthesis of Phytosterols’ Esters
4. Biocatalysis in the Synthesis of Phytosterol Esters
4.1. General Aspects
4.2. Parameters Affecting the Biocatalytic Synthesis of Phytosterols’ Esters
4.2.1. Effect of Temperature
4.2.2. Effect of Substrate Molar Ratio
4.2.3. Enzyme Source and Load
4.2.4. Effect of Reaction Medium
4.2.5. Reaction Time
4.3. Potential Technologies for Biocatalytic Synthesis of Phytosterol Esters
5. Analytical Methods for Phytosterol Esters’ Detection: Confirming the Synthesis
6. Technology Challenges and Safety of Sterol Esters
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daels, E.; Foubert, I.; Goderis, B. The effect of adding a commercial phytosterol ester mixture on the phase behavior of palm oil. Food Res. Int. 2017, 100, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Lagarda, M.J.; García-Llatas, G.; Farré, R. Analysis of phytosterols in foods. J. Pharm. Biomed. Anal. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, P.A.; Ballus, C.A.; Teixeira-Filho, J.; Godoy, H.T. Phytosterols and tocopherols content of pulps and nuts of Brazilian fruits. Food Res. Int. 2010, 43, 1603–1606. [Google Scholar] [CrossRef]
- Santos, R.; Limas, E.; Sousa, M.; da Conceição Castilho, M.; Ramos, F.; da Silveira, M.I.N. Optimization of analytical procedures for GC-MS determination of phytosterols and phytostanols in enriched milk and yoghurt. Food Chem. 2007, 102, 113–117. [Google Scholar] [CrossRef]
- Pennisi Forell, S.C.; Ranalli, N.; Zaritzky, N.E.; Andrés, S.C.; Califano, A.N. Effect of type of emulsifiers and antioxidants on oxidative stability, colour and fatty acid profile of low-fat beef burgers enriched with unsaturated fatty acids and phytosterols. Meat Sci. 2010, 86, 364–370. [Google Scholar] [CrossRef]
- Brufau, G.; Canela, M.A.; Rafecas, M. Phytosterols: Physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr. Res. 2008, 28, 217–225. [Google Scholar] [CrossRef]
- Toivo, J.; Phillips, K.; Lampi, A.M.; Piironen, V. Determination of sterols in foods: Recovery of free, esterified, and glycosidic sterols. J. Food Compos. Anal. 2001, 14, 631–643. [Google Scholar] [CrossRef]
- Mel’nikov, S.M.; Seijen Ten Hoorn, J.W.M.; Eijkelenboom, A.P.A.M. Effect of phytosterols and phytostanols on the solubilization of cholesterol by dietary mixed micelles: An in vitro study. Chem. Phys. Lipids 2004, 127, 121–141. [Google Scholar] [CrossRef]
- Rozner, S.; Popov, I.; Uvarov, V.; Aserin, A.; Garti, N. Templated cocrystallization of cholesterol and phytosterols from microemulsions. J. Cryst. Growth 2009, 311, 4022–4033. [Google Scholar] [CrossRef]
- Binder, T.P.; Gottemoller, T.V. Hydrothermically Processed Compositions Containing Phytosterols. U.S. Patent Application 10/410,193, 22 January 2004. [Google Scholar]
- Di Battista, C.A.; Ramírez-Rigo, M.V.; Piña, J. Microencapsulation of Phytosterols by Spray Drying. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 56, pp. 437–468. [Google Scholar]
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.L.; Burrin, D.G.; Guthrie, G.; Thijs, C.; Te Velde, A.A.; Vreugdenhil, A.C.E.; et al. Plant-based sterols and stanols in health & disease: “Consequences of human development in a plant-based environment?”. Prog. Lipid Res. 2019, 74, 87–102. [Google Scholar] [CrossRef]
- Yang, F.; Oyeyinka, S.A.; Ma, Y. Novel Synthesis of Phytosterol Ester from Soybean Sterol and Acetic Anhydride. J. Food Sci. 2016, 81, C1629–C1635. [Google Scholar] [CrossRef]
- Wester, I. Cholesterol-lowering effect of plant sterols. Eur. J. Lipid Sci. Technol. 2000, 102, 37–44. [Google Scholar] [CrossRef]
- Tan, Z.; Shahidi, F. Optimization of enzymatic synthesis of phytosteryl caprylates using response surface methodology. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 657–666. [Google Scholar] [CrossRef]
- He, W.S.; Li, L.L.; Huang, Q.J.; Yin, J.; Cao, X.C. Highly efficient synthesis of phytosterol linolenate in the presence of Bronsted acidic ionic liquid. Food Chem. 2018, 263, 1–7. [Google Scholar] [CrossRef]
- Yang, F.; Oyeyinka, S.A.; Xu, W.; Ma, Y.; Zhou, S. In vitro bioaccessibility and physicochemical properties of phytosterol linoleic ester synthesized from soybean sterol and linoleic acid. LWT-Food Sci. Technol. 2018, 92, 265–271. [Google Scholar] [CrossRef]
- Mussner, M.J.; Parhofer, K.G.; Von Bergmann, K.; Schwandt, P.; Broedl, U.; Otto, C. Effects of phytosterol ester-enriched margarine on plasma lipoproteins in mild to moderate hypercholesterolemia are related to basal cholesterol and fat intake. Metabolism 2002, 51, 189–194. [Google Scholar] [CrossRef]
- Davidson, M.H.; Maki, K.C.; Umporowicz, D.M.; Ingram, K.A.; Dicklin, M.R.; Lane, R.W.; Franke, W.C.; Robins, S.J.; Schaefer, E.; McNamara, J.R.; et al. Safety and tolerability of esterified phytosterols administered in reduced-fat spread and salad dressing to healthy adult men and women. J. Am. Coll. Nutr. 2001, 20, 307–319. [Google Scholar] [CrossRef]
- Kwak, H.S.; Ahn, H.J.; Ahn, J. Development of phytosterol ester-added Cheddar cheese for lowering blood cholesterol. Asian-Australas. J. Anim. Sci. 2005, 18, 267–276. [Google Scholar] [CrossRef]
- Meng, X.; Sun, P.; Pan, Q.; Shi, Z.; Yang, K.; He, R. Synthesis of plant sterol esters catalyzed by heteropolyacid in a solvent-free system. Eur. J. Lipid Sci. Technol. 2006, 108, 13–18. [Google Scholar] [CrossRef]
- Pouilloux, Y.; Courtois, G.; Boisseau, M.; Piccirilli, A.; Barrault, J. Solid base catalysts for the synthesis of phytosterol esters. Green Chem. 2003, 5, 89–91. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Huang, K.C.; Su, C.H. Green process for the preparation of phytosterol esters: Microwave-mediated noncatalytic synthesis. Chem. Eng. J. 2020, 382, 122796. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Kodali, S.; Balle, T.; Chen, B.; Guo, Z. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresour. Technol. 2017, 224, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brígida, A.I.S.; Amaral, P.F.F.; Coelho, M.A.Z.; Gonçalves, L.R.B. Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2014, 101, 148–158. [Google Scholar] [CrossRef]
- Akil, E.; Pereira, A.d.S.; El-Bacha, T.; Amaral, P.F.F.; Torres, A.G. Efficient production of bioactive structured lipids by fast acidolysis catalyzed by Yarrowia lipolytica lipase, free and immobilized in chitosan-alginate beads, in solvent-free medium. Int. J. Biol. Macromol. 2020, 163, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Pohl, M. Improved biocatalysts by directed evolution and rational protein design. Curr. Opin. Chem. Biol. 2001, 5, 137–143. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Fraga, J.L.; Penha, A.C.B.; Pereira, A.d.S.; Silva, K.A.; Akil, E.; Torres, A.G.; Amaral, P.F.F. Use of Yarrowia lipolytica Lipase Immobilized in Cell Debris for the Production of Lipolyzed Milk Fat (LMF). Int. J. Mol. Sci. 2018, 19, 3413. [Google Scholar] [CrossRef] [Green Version]
- Vilas Bôas, R.N.; Ceron, A.A.; Bento, H.B.S.; de Castro, H.F. Application of an immobilized Rhizopus oryzae lipase to batch and continuous ester synthesis with a mixture of a lauric acid and fusel oil. Biomass Bioenergy 2018, 119, 61–68. [Google Scholar] [CrossRef]
- Yang, T.; Fruekilde, M.B.; Xu, X. Applications of Immobilized Thermomyces Ianuginosa Lipase in Interesterification. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 881–887. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Effect of water on lipase NS81006-catalyzed alcoholysis for biodiesel production. Process Biochem. 2017, 58, 239–244. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, J.; Anankanbil, S.; Chen, M.; Guo, Z.; Adams, J.P.; Snajdrova, R.; Li, Z. Amide Synthesis via Aminolysis of Ester or Acid with an Intracellular Lipase. ACS Catal. 2018, 8, 8856–8865. [Google Scholar] [CrossRef]
- Rajendran, A.; Palanisamy, A.; Thangavelu, V. Lipase catalyzed ester synthesis for food processing industries. Braz. Arch. Biol. Technol. 2009, 52, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Casas-Godoy, L.; Gasteazoro, F.; Duquesne, S.; Bordes, F.; Marty, A.; Sandoval, G. Lipases: An Overview. Methods Mol. Biol. 2018, 1835, 3–38. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Pereira, A.d.S.; Fontes-Sant’Ana, G.C.; Amaral, P.F.F. Mango agro-industrial wastes for lipase production from Yarrowia lipolytica and the potential of the fermented solid as a biocatalyst. Food Bioprod. Process. 2019, 115, 68–77. [Google Scholar] [CrossRef]
- He, Y.Q.; Tan, T.W. Use of response surface methodology to optimize culture medium for production of lipase with Candida sp. 99-125. J. Mol. Catal. B Enzym. 2006, 43, 9–14. [Google Scholar] [CrossRef]
- Fraga, J.L.; Souza, C.P.L.; Pereira, A.d.S.; Aguieiras, E.C.G.; de Silva, L.O.; Torres, A.G.; Freire, D.G.; Amaral, P.F.F. Palm oil wastes as feedstock for lipase production by Yarrowia lipolytica and biocatalyst application/reuse. 3 Biotech 2021, 11, 191. [Google Scholar] [CrossRef]
- Nunes, P.M.B.; Fraga, J.L.; Ratier, R.B.; Rocha-Leão, M.H.M.; Brígida, A.I.S.; Fickers, P.; Amaral, P.F.F. Waste soybean frying oil for the production, extraction, and characterization of cell-wall-associated lipases from Yarrowia lipolytica. Bioprocess Biosyst. Eng. 2021, 44, 809–818. [Google Scholar] [CrossRef]
- Pereira, A.d.S.; Diniz, M.M.; De Jong, G.; Gama Filho, H.S.; dos Anjos, M.J.; Finotelli, P.V.; Fontes-Sant’Ana, G.C.; Amaral, P.F.F. Chitosan-alginate beads as encapsulating agents for Yarrowia lipolytica lipase: Morphological, physico-chemical and kinetic characteristics. Int. J. Biol. Macromol. 2019, 139, 621–630. [Google Scholar] [CrossRef]
- Pereira, A.d.S.; Fraga, J.L.; Diniz, M.M.; Fontes-Sant’ana, G.C.; Amaral, P.F.F. High catalytic activity of lipase from Yarrowia lipolytica immobilized by microencapsulation. Int. J. Mol. Sci. 2018, 19, 3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, D.N.; Balkus, K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Carvalho, T.; Pereira, A.d.S.; Bonomo, R.C.F.; Franco, M.; Finotelli, P.V.; Amaral, P.F.F. Simple physical adsorption technique to immobilize Yarrowia lipolytica lipase purified by different methods on magnetic nanoparticles: Adsorption isotherms and thermodynamic approach. Int. J. Biol. Macromol. 2020, 160, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Lenfant, N.; Hotelier, T.; Velluet, E.; Bourne, Y.; Marchot, P.; Chatonnet, A. ESTHER, the database of the α/β-hydrolase fold superfamily of proteins: Tools to explore diversity of functions. Nucleic Acids Res. 2013, 41, D423–D429. [Google Scholar] [CrossRef] [Green Version]
- Anobom, C.D.; Pinheiro, A.S.; De-Andrade, R.A.; Aguieiras, E.C.G.; Andrade, G.C.; Moura, M.V.; Almeida, R.V.; Freire, D.M. From structure to catalysis: Recent developments in the biotechnological applications of lipases. BioMed Res. Int. 2014, 2014, 684506. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Kumari, A.; Syal, P.; Singh, Y. Molecular and functional diversity of yeast and fungal lipases: Their role in biotechnology and cellular physiology. Prog. Lipid Res. 2015, 57, 40–54. [Google Scholar] [CrossRef]
- Pleiss, J.; Fischer, M.; Schmid, R.D. Anatomy of lipase binding sites: The scissile fatty acid binding site. Chem. Phys. Lipids 1998, 93, 67–80. [Google Scholar] [CrossRef]
- De Almeida, J.M.; Moure, V.R.; Müller-Santos, M.; De Souza, E.M.; Pedrosa, F.O.; Mitchell, D.A.; Krieger, N. Tailoring recombinant lipases: Keeping the His-tag favors esterification reactions, removing it favors hydrolysis reactions. Sci. Rep. 2018, 8, 10000. [Google Scholar] [CrossRef]
- Cen, Y.; Singh, W.; Arkin, M.; Moody, T.S.; Huang, M.; Zhou, J.; Wu, Q.; Reetz, M.T. Artificial cysteine-lipases with high activity and altered catalytic mechanism created by laboratory evolution. Nat. Commun. 2019, 10, 3198. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef]
- Bassegoda, A.; Cesarini, S.; Diaz, P. Lipase improvement: Goals and strategies. Comput. Struct. Biotechnol. J. 2012, 2, e201209005. [Google Scholar] [CrossRef] [Green Version]
- Quin, M.B.; Schmidt-Dannert, C. Engineering of biocatalysts: From evolution to creation. ACS Catal. 2011, 1, 1017–1021. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Suen, W.C.; Windsor, W.; Xiao, L.; Madison, V.; Zaks, A. Improving tolerance of Candida antarctica lipase B towards irreversible thermal inactivation through directed evolution. Protein Eng. 2003, 16, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Elgharbawy, A.A.; Riyadi, F.A.; Alam, M.Z.; Moniruzzaman, M. Ionic liquids as a potential solvent for lipase-catalysed reactions: A review. J. Mol. Liq. 2018, 251, 150–166. [Google Scholar] [CrossRef]
- Duhan, N.; Barak, S.; Mudgil, D. Bioactive lipids: Chemistry & health benefits. Biointerface Res. Appl. Chem. 2020, 10, 6676–6687. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Bari, M.; Bisogno, T.; Battista, N. Bioactive lipids in health and disease. Biomolecules 2020, 10, 1698. [Google Scholar] [CrossRef]
- O’Shea, M.; Bassaganya-Riera, J.; Mohede, I.C.M. Immunomodulatory properties of conjugated linoleic acid. Am. J. Clin. Nutr. 2004, 79, 1199–1206. [Google Scholar] [CrossRef] [Green Version]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, S.G.; Olaniru, O.B. A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Pelagia Res. Libr. Asian J. Plant Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.S.; Cui, D.D.; Zhang, Y.L.; Liu, Y.; Yin, J.; Chen, G.; Jia, C.S.; Feng, B. Highly efficient synthesis of phytosterol linolenate catalyzed by Candida rugosa lipase through transesterification. Food Sci. Technol. Res. 2017, 23, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Akashe, A.; Miller, M. Use of Mesophase-Stabilized Compositions for Delivery of Cholesterol-Reducing Sterols and Stanols in Food Products. U.S. Patent 6,274,574, 14 August 2001. [Google Scholar]
- He, W.S.; Zhu, H.; Chen, Z.Y. Plant Sterols: Chemical and Enzymatic Structural Modifications and Effects on Their Cholesterol-Lowering Activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Meng, X.; Pan, Q.; Yang, T. Synthesis of phytosteryl esters by using alumina-supported zinc oxide (ZnO/Al2O3) from esterification production of phytosterol with fatty acid. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 143–149. [Google Scholar] [CrossRef]
- He, B.; Deng, T.; Li, J.; Yan, F.; Wang, H.; Huang, Y.; Peng, C. An innovative auto-catalytic esterification for the production of phytosterol esters: Experiment and kinetics. RSC Adv. 2014, 4, 64319–64327. [Google Scholar] [CrossRef]
- Saroja, M.; Kaimal, T.N.B. A convenient method of esterification of fatty acids. Preparation of alkyl esters, sterol esters, wax esters and triacylglycerols. Synth. Commun. 1986, 16, 1423–1430. [Google Scholar] [CrossRef]
- SÁ, A.G.A.; de Meneses, A.C.; de Araújo, P.H.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Ansorge-Schumacher, M.B.; Thum, O. Immobilised lipases in the cosmetics industry. Chem. Soc. Rev. 2013, 42, 6475–6490. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Fu, Z.; Wei, G.; Li, H.; Zhang, B.; Zheng, L.; Deng, Z. Enzymatic Synthesis of β-Sitosterol Laurate by Candida rugosa Lipase AY30 in the Water/AOT/Isooctane Reverse Micelle. Appl. Biochem. Biotechnol. 2020, 192, 392–414. [Google Scholar] [CrossRef]
- Yao, G.; Wang, X.; Yang, M.; Chen, F.; Ling, Y.; Liu, T.; Xing, S.; Yao, M.; Zhang, F. Co-immobilization of bi-lipases on magnetic nanoparticles as an efficient catalyst for synthesis of functional oil rich in diacylglycerols, phytosterol esters and α-linolenic acid. LWT 2020, 129, 109522. [Google Scholar] [CrossRef]
- Chang, M.; Zhang, T.; Feng, W.; Wang, T.; Liu, R.; Jin, Q.; Wang, X. Preparation of highly pure stigmasteryl oleate by enzymatic esterification of stigmasterol enriched from soybean phytosterols. LWT 2020, 128, 109464. [Google Scholar] [CrossRef]
- Yu, D.; Wang, T.; Chen, J.; Tang, H.; Li, D.; Zhang, X.; Geng, H.; Wang, L.; Elfalleh, W.; Jiang, L. Enzymatic esterification of rice bran oil and phytosterol in supercritical CO2. J. Food Process. Preserv. 2019, 43, e14066. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Liu, P.; An, N.; Chen, G.; Zhao, R.; Hang, Z. Catalytic synthesis of β-sitosterol linolenate by Pickering emulsion-immobilized lipase. In E3S Web of Conferences; EDP Sciences: Ulis, France, 2019; Volume 78, p. 02019. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, M.Y.; Shi, J.; Zheng, M.M.; Huang, F.H. Preparation of immobilized lipase based on hollow mesoporous silica spheres and its application in ester synthesis. Molecules 2019, 24, 395. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Zhang, X.; Zou, D.; Wang, T.; Liu, T.; Wang, L.; Elfalleh, W.; Jiang, L. Immobilized CALB Catalyzed Transesterification of Soybean Oil and Phytosterol. Food Biophys. 2018, 13, 208–215. [Google Scholar] [CrossRef]
- Choi, N.; Cho, H.J.; Kim, H.; Kim, Y.; Kim, H.R.; Kim, I.H. Preparation of phytosteryl ester and simultaneous enrichment of stearidonic acid via lipase-catalyzed esterification. Process Biochem. 2017, 61, 88–94. [Google Scholar] [CrossRef]
- Shang, C.Y.; Li, W.X.; Zhang, R.F. Immobilization of Candida rugosa lipase on ZnO nanowires/macroporous silica composites for biocatalytic synthesis of phytosterol esters. Mater. Res. Bull. 2015, 68, 336–342. [Google Scholar] [CrossRef]
- Cui, C.; Guan, N.; Xing, C.; Chen, B.; Tan, T. Immobilization of Yarrowia lipolytica lipase Ylip2 for the biocatalytic synthesis of phytosterol ester in a water activity controlled reactor. Colloids Surfaces B Biointerfaces 2016, 146, 490–497. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, J.; Zeng, A. Optimization and modeling for the synthesis of sterol esters from deodorizer distillate by lipase-catalyzed esterification. Biotechnol. Appl. Biochem. 2017, 64, 270–278. [Google Scholar] [CrossRef]
- Choi, N.; Kim, H.; Kim, B.H.; Lee, J.; Kim, I.H. Production of phytosteryl ester from echium oil in a recirculating packed bed reactor using an immobilized lipase. J. Oleo Sci. 2017, 66, 1329–1335. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Qi, S.; Li, Z.; Luo, R.; Yang, B.; Wang, Y. Enzymatic synthesis of phytosterol esters catalyzed by Candida rugosa lipase in water-in-[Bmim]PF6 microemulsion. Bioprocess Biosyst. Eng. 2015, 38, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.Y.; Li, W.X.; Jiang, F.; Zhang, R.F. Improved enzymatic properties of Candida rugosa lipase immobilized on ZnO nanowires/macroporous SiO2 microwave absorbing supports. J. Mol. Catal. B Enzym. 2015, 113, 9–13. [Google Scholar] [CrossRef]
- Zheng, M.M.; Huang, Q.; Huang, F.H.; Guo, P.M.; Xiang, X.; Deng, Q.C.; Li, W.L.; Wan, C.Y.; Zheng, C. Production of novel “functional oil” rich in diglycerides and phytosterol esters with “one-pot” enzymatic transesterification. J. Agric. Food Chem. 2014, 62, 5142–5148. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liao, J.; Jiang, S.; Zuo, Y. Lipase-catalyed synthesis phytosterol oleic acid ester by microwave. J. Chin. Cereal. Oils Assoc. 2014, 29, 95–100. [Google Scholar]
- Kobayashi, T.; Ogino, A.; Miyake, Y.; Mori, H.; Hosoda, A.; Fujita, M.; Tsuno, T.; Adachi, S. Lipase-catalyzed esterification of triterpene alcohols and phytosterols with oleic acid. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1885–1890. [Google Scholar] [CrossRef] [Green Version]
- No, D.S.; Zhao, T.; Lee, J.; Lee, J.S.; Kim, I.H. Synthesis of phytosteryl ester containing pinolenic acid in a solvent-free system using immobilized Candida rugosa lipase. J. Agric. Food Chem. 2013, 61, 8934–8940. [Google Scholar] [CrossRef]
- Panpipat, W.; Xu, X.; Guo, Z. Improved acylation of phytosterols catalyzed by Candida antarctica lipase A with superior catalytic activity. Biochem. Eng. J. 2013, 70, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.M.; Lu, Y.; Huang, F.H.; Wang, L.; Guo, P.M.; Feng, Y.Q.; Deng, Q.C. Lipase immobilization on hyper-cross-linked polymer-coated silica for biocatalytic synthesis of phytosterol esters with controllable fatty acid composition. J. Agric. Food Chem. 2013, 61, 231–237. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, M.; Ren, L.; Zhou, H.; Wei, P. Synthesis of phytosterol esters catalyzed by immobilized lipase in organic media. Cuihua Xuebao/Chin. J. Catal. 2013, 34, 2255–2262. [Google Scholar] [CrossRef]
- Pan, X.; Chen, B.; Wang, J.; Zhang, X.; Zhul, B.; Tan, T. Enzymatic synthesizing of phytosterol oleic esters. Appl. Biochem. Biotechnol. 2012, 168, 68–77. [Google Scholar] [CrossRef]
- Zheng, M.M.; Lu, Y.; Dong, L.; Guo, P.M.; Deng, Q.C.; Li, W.L.; Feng, Y.Q.; Huang, F.H. Immobilization of Candida rugosa lipase on hydrophobic/strong cation-exchange functional silica particles for biocatalytic synthesis of phytosterol esters. Bioresour. Technol. 2012, 115, 141–146. [Google Scholar] [CrossRef]
- Torrelo, G.; Torres, C.F.; Reglero, G. Enzymatic strategies for solvent-free production of short and medium chain phytosteryl esters. Eur. J. Lipid Sci. Technol. 2012, 114, 670–676. [Google Scholar] [CrossRef]
- Tan, Z.; Shahidi, F. A novel chemoenzymatic synthesis of phytosteryl caffeates and assessment of their antioxidant activity. Food Chem. 2012, 133, 1427–1434. [Google Scholar] [CrossRef]
- Zheng, M.M.; Dong, L.; Lu, Y.; Guo, P.M.; Deng, Q.C.; Li, W.L.; Feng, Y.Q.; Huang, F.H. Immobilization of Candida rugosa lipase on magnetic poly(allyl glycidyl ether-co-ethylene glycol dimethacrylate) polymer microsphere for synthesis of phytosterol esters of unsaturated fatty acids. J. Mol. Catal. B Enzym. 2012, 74, 16–23. [Google Scholar] [CrossRef]
- Fauré, N.; Illanes, A. Immobilization of Pseudomonas stutzeri lipase for the transesterification of wood sterols with fatty acid esters. Appl. Biochem. Biotechnol. 2011, 165, 1332–1341. [Google Scholar] [CrossRef]
- Hellner, G.; Toke, E.R.; Nagy, V.; Szakács, G.; Poppe, L. Integrated enzymatic production of specific structured lipid and phytosterol ester compositions. Process Biochem. 2010, 45, 1245–1250. [Google Scholar] [CrossRef]
- Sengupta, A.; Pal, M.; Silroy, S.; Ghosh, M. Comparative study of sterol ester synthesis using thermomyces lanuginosus lipase in stirred tank and packed-bed bioreactors. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 1019–1025. [Google Scholar] [CrossRef]
- Torres, C.F.; Torrelo, G.; Vazquez, L.; Señorans, F.J.; Reglero, G. Stepwise Esterification of Phytosterols with Conjugated Linoleic Acid Catalyzed by Candida rugosa Lipase in Solvent-free Medium. J. Biosci. Bioeng. 2008, 106, 559–562. [Google Scholar] [CrossRef]
- Torrelo, G.; Torres, C.F.; Señorans, F.J.; Blanco, R.M.; Reglero, G. Solvent-free preparation of phytosteryl esters with fatty acids from butterfat in equimolecular conditions in the presence of a lipase from Candida rugosa. J. Chem. Technol. Biotechnol. 2009, 84, 745–750. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Modeling and optimization of lipase-catalyzed synthesis of phytosteryl esters of oleic acid by response surface methodology. Food Chem. 2007, 102, 336–342. [Google Scholar] [CrossRef]
- Villeneuve, P.; Turon, F.; Caro, Y.; Escoffier, R.; Baréa, B.; Barouh, B.; Lago, R.; Piombo, G.; Pina, M. Lipase-catalyzed synthesis of canola phytosterols oleate esters as cholesterol lowering agents. Enzyme Microb. Technol. 2005, 37, 150–155. [Google Scholar] [CrossRef]
- Vu, P.L.; Shin, J.A.; Lim, C.H.; Lee, K.T. Lipase-catalyzed production of phytosteryl esters and their crystallization behavior in corn oil. Food Res. Int. 2004, 37, 175–180. [Google Scholar] [CrossRef]
- Negishi, S.; Hidaka, I.; Takahashi, I.; Kunita, S. Transesterification of Phytosterol and Edible Oil by Lipase Powder at High Temperature. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 905–907. [Google Scholar] [CrossRef]
- Raja Rajan, R.G.; Gopala Krishna, A.G. A Simple Process for the Enzymatic Synthesis of Phytosterol Esters of Alpha-Linolenic Acid. Eur. J. Lipid Sci. Technol. 2014, 46, 131–137. [Google Scholar]
- Torres, C.F.; Torrelo, G.; Señorans, F.J.; Reglero, G. A two steps enzymatic procedure to obtain sterol esters, tocopherols and fatty acid ethyl esters from soybean oil deodorizer distillate. Process Biochem. 2007, 42, 1335–1341. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Huang, F.; Zheng, M. An efficient and robust continuous-flow bioreactor for the enzymatic preparation of phytosterol esters based on hollow lipase microarray. Food Chem. 2022, 372, 131256. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, H.K. Antibacterial activity of emulsions containing unsaturated fatty acid ergosterol esters synthesized by lipase-mediated transesterification. Enzyme Microb. Technol. 2020, 139, 109581. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Wang, M.Y.; Song, Y.Q.; Hu, L.Z.; Yu, D.Y. Applying Response Surface Methodology to Optimize the Lipase-catalyzed Synthesis of Phytosterol Ester. Food Sci. 2011, 32, 59. [Google Scholar] [CrossRef]
- Liu, H.-L.; Miao, M.; Jiang, B.; Zhang, T. Bio-synthesis of Phytosterol Laurate in Non-aqueous Phase Reaction. Food Ferment. Ind. 2011, 37, 37–41. [Google Scholar] [CrossRef]
- He, W.-S.; Li, L.; Zhao, J.; Xu, H.; Rui, J.; Cui, D.; Li, H.; Zhang, H.; Liu, X. Candida sp. 99-125 lipase-catalyzed synthesis of ergosterol linolenate and its characterization. Food Chem. 2019, 280, 286–293. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Shi, J.; Tang, H.; Xiang, X.; Huang, F.; Zheng, M. Carbon Nanoparticle-Stabilized Pickering Emulsion as a Sustainable and High-Performance Interfacial Catalysis Platform for Enzymatic Esterification/Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 7619–7629. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, M.; Liu, Z.; Shi, J.; Huang, F.; Luo, X. Constructing a Continuous Flow Bioreactor Based on a Hierarchically Porous Cellulose Monolith for Ultrafast and Nonstop Enzymatic Esterification/Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 2056–2063. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, J.; Huang, F.; Xiang, X.; Shi, J.; Deng, Q.; Ma, F.; Feng, Y. Enzymatic deacidification of the rice bran oil and simultaneous preparation of phytosterol esters-enriched functional oil catalyzed by immobilized lipase arrays. RSC Adv. 2015, 5, 70073–70079. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, B.; Yang, G.; Chen, J.; Liu, W. Enzymatic preparation of phytosterol esters with fatty acids from high-oleic sunflower seed oil using response surface methodology. RSC Adv. 2021, 11, 15204–15212. [Google Scholar] [CrossRef]
- Li, P.; Liu, G.; Chen, J.; Li, L. Enzymatic synthesis of phytosterol esters with pyruvate and phytosterol. China Oils Fats 2012, 10. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-ZYZZ201210010.htm (accessed on 16 November 2021).
- Tan, Z.; Le, K.; Moghadasian, M.; Shahidi, F. Enzymatic synthesis of phytosteryl docosahexaneates and evaluation of their anti-atherogenic effects in apo-E deficient mice. Food Chem. 2012, 134, 2097–2104. [Google Scholar] [CrossRef]
- Wang, H.; Jia, C.; Xia, X.; Karangwa, E.; Zhang, X. Enzymatic synthesis of phytosteryl lipoate and its antioxidant properties. Food Chem. 2018, 240, 736–742. [Google Scholar] [CrossRef]
- Schär, A.; Liphardt, S.; Nyström, L. Enzymatic synthesis of steryl hydroxycinnamates and their antioxidant activity. Eur. J. Lipid Sci. Technol. 2017, 119, 1600267. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Wang, T.; Geng, H.; Wang, L.; Jiang, L.; Elfalleh, W. Immobilized Candida antarctica lipase B (CALB) on functionalized MCM-41: Stability and catalysis of transesterification of soybean oil and phytosterol. Food Biosci. 2021, 40, 100906. [Google Scholar] [CrossRef]
- Miao, M.; Liu, H.; Jiang, B.; Yang, C.; Xia, X.; Zhang, T. Enzyme-catalysed synthesis of plant steryl laurate in non-aqueous media using salt hydrate pairs and its characterisation. J. Funct. Foods 2014, 7, 452–461. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.M. Lipase2catalyzed synthesis of β-sitosterol ester with conjugated linoleic acid in organic solvent. China Oils Fats 2006, 31. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-ZYZZ200602018.htm (accessed on 28 November 2021).
- Yang, T.; Zhang, Y.; Wang, J.; Huang, F.; Zheng, M. Magnetic Switchable Pickering Interfacial Biocatalysis: One-Pot Cascade Synthesis of Phytosterol Esters from High-Acid Value Oil. ACS Sustain. Chem. Eng. 2021, 9, 12070–12078. [Google Scholar] [CrossRef]
- Rui, L.; Jia, C.S.; Lin, Y.; Zhang, X.M.; Xia, Q.Y.U.; Zhao, S.L.; Biao, F.; Fang, Z.A.; Chen, W.J. Lipase-Catalyzed Synthesis of Conjugated Linoleyl β-Sitosterol and Its Cholesterol-Lowering Properties in Mice. J. Agric. Food Chem. 2010, 58, 1898–1902. [Google Scholar] [CrossRef]
- Luo, R.; Zeng, C.; Xu, D.; Chen, H. Lipase-catalyzed synthesis of phytosterol myristate. Sci. Technol. Food Ind. 2012, 9. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-SPKJ201209036.htm (accessed on 28 November 2021).
- Mao, Y.; Xu, D.; Yang, B.; Chen, H.; Zhao, J. Lipase-catalyzed synthesis of phytosterol palmitate esters in organic media. China Oils Fats 2011, 12. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-ZYZZ201112008.htm (accessed on 28 November 2021).
- Hu, L.; Llibin, S.; Li, J.; Qi, L.; Zhang, X.; Yu, D.; Walid, E.; Jiang, L. Lipase-catalyzed transesterification of soybean oil and phytosterol in supercritical CO2. Bioprocess Biosyst. Eng. 2015, 38, 2343–2347. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, H.; Geng, C.; Han, Y. Optimization of Lipase catalyzed synthesis of conjugated linoleic acid phytosterol ester. J. Northwest A F Univ.-Nat. Sci. Ed. 2014, 42, 173–179. [Google Scholar]
- Zhu, Z.; Xu, L.; Zhang, H.; Yan, Y. Optimization of Candida rugosa lipase catalyzed synthesis of sterol conjugated linoleic acid ester by response surface methodology. China Oils Fats 2013, 7. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-ZYZZ201307013.htm (accessed on 28 November 2021).
- Li, J.; Deng, Q.; Zhang, P.; Huang, F. Research on synthesis process of phytosterols α-linolenate by catalysis of lipase. Sci. Technol. Food Ind 2008, 12, 128–131. [Google Scholar]
- Yu, D.; Yu, C.; Wang, T.; Chen, J.; Zhang, X.; Wang, L.; Qin, L.; Wu, F. Study on the Deacidification of Rice Bran Oil Esterification by Magnetic Immobilized Lipase. Catal. Lett. 2020, 150, 1256–1267. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, Z.; Ren, L.; Yu, M.; Zhou, H.; Wei, P. Synthesis of Phytosterol Esters Catalyzed by Immobilized Lipase in Solvent-free System. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-HGYJ201603022.htm (accessed on 16 November 2021).
- Sengupta, A.; Ghosh, M. The kinetics of enzyme catalyzed synthesis of sterol ester. Eur. J. Lipid Sci. Technol. 2011, 113, 763–767. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, L.; Huang, F.H.; Dong, L.; Guo, P.M.; Deng, Q.C.; Li, W.L.; Zheng, C. Ultrasonic pretreatment for lipase-catalyed synthesis of phytosterol esters with different acyl donors. Ultrason. Sonochem. 2012, 19, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Domínguez De María, P.; Sánchez-Montero, J.M.; Sinisterra, J.V.; Alcántara, A.R. Understanding Candida rugosa lipases: An overview. Biotechnol. Adv. 2006, 24, 180–196. [Google Scholar] [CrossRef]
- Tan, Z.; Shahidi, F. Phytosteryl sinapates and vanillates: Chemoenzymatic synthesis and antioxidant capacity assessment. Food Chem. 2013, 138, 1438–1447. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, M.; Shi, W.; Xiang, X.; Shi, J.; Deng, Q.; Li, W.; Wan, C. Method for Preparing Functional Edible Oil Rich in Phytosterol Esters and Diglycerides. U.S. Patent 10,258,058, 16 April 2019. [Google Scholar]
- Kim, I.-H.; Nodasom; Jo, J.-J. Preparation Method of Fatty Acid Phytosterol Ester Using Immobilized Lipase Derived from Candida Rugosa. Korean Patent KR20150046397A, 30 April 2015. [Google Scholar]
- Lillan, J.T.; Borch, S.J. Method for Producing Phytosterol/Phytostanol Phospholipid Esters. U.S. Patent 13/231,355, 7 June 2012. [Google Scholar]
- Hu, X.; Chen, H.; Jiang, M.; Dong, X.; Liu, C.; Wei, F.; Zhang, Y.; Huang, F. Method for Producing Plant Sterol Ester by Immobilized Whole-Cell Enzyme Catalysis in Solvent-Free System. CN Patent CN101200754B, 2 June 2010. [Google Scholar]
- Zhang, Z.; Fan, L.-P.; Wang, Y.-J. Applications of Chemical Kinetics in Heterogeneous Catalysis. In Advanced Oxidation Processes—Applications, Trends, and Prospects; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Yahya, A.R.M.; Anderson, W.A.; Moo-Young, M. Ester synthesis in lipase-catalyzed reactions. Enzyme Microb. Technol. 1998, 23, 438–450. [Google Scholar] [CrossRef]
- Handayani, S.; Tamara Putri, A.T.; Setiasih, S.; Hudiyono, S. Enzymatic Synthesis of Glyserol-Coconut Oil Fatty Acid and Glycerol-Decanoic Acis Ester as Emulsifier and Antimicrobial Agents Using Candida rugosa Lipase EC 3.1.1.3. In Conference Proceeding of The 3rd Asia-Pacific Conference on Life Science and Engineering (APCLSE); IOP Publishing: Bristol, UK, 2018; Volume 299, p. 12019. [Google Scholar] [CrossRef]
- Senoymak Tarakcı, M.I.; Ilgen, O. Esterification of Oleic Acid with Methanol Using Zr(SO4)2 as a Heterogeneous Catalyst. Chem. Eng. Technol. 2018, 41, 845–852. [Google Scholar] [CrossRef]
- Hernáiz, M.J.; Sánchez-Montero, J.M.; Sinisterra, J.V. New differences between isoenzymes A and B from Candida rugosa lipase. Biotechnol. Lett. 1997, 19, 303–306. [Google Scholar] [CrossRef]
- Molina-Gutiérrez, M.; Hakalin, N.L.S.; Rodríguez-Sanchez, L.; Prieto, A.; Martínez, M.J. Green synthesis of β-sitostanol esters catalyzed by the versatile lipase/sterol esterase from Ophiostoma piceae. Food Chem. 2017, 221, 1458–1465. [Google Scholar] [CrossRef]
- Khan, N.R.; Rathod, V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process Biochem. 2015, 50, 1793–1806. [Google Scholar] [CrossRef]
- Ben Akacha, N.; Gargouri, M. Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food Bioprod. Process. 2015, 94, 675–706. [Google Scholar] [CrossRef]
- Patel, V.; Gajera, H.; Gupta, A.; Manocha, L.; Madamwar, D. Synthesis of ethyl caprylate in organic media using Candida rugosa lipase immobilized on exfoliated graphene oxide: Process parameters and reusability studies. Biochem. Eng. J. 2015, 95, 62–70. [Google Scholar] [CrossRef]
- Klibanov, A.M. Asymmetric Transformations Catalyzed by Enzymes in Organic Solvents. Acc. Chem. Res. 1990, 23, 114–120. [Google Scholar] [CrossRef]
- Castro-Ochoa, L.D.; Rodríguez-Gómez, C.; Valerio-Alfaro, G.; Oliart Ros, R. Screening, purification and characterization of the thermoalkalophilic lipase produced by Bacillus thermoleovorans CCR11. Enzyme Microb. Technol. 2005, 37, 648–654. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Khmelnitsky, Y.L.; Levashov, A.V.; Klyachko, N.L.; Martinek, K. Engineering biocatalytic systems in organic media with low water content. Enzyme Microb. Technol. 1988, 10, 710–724. [Google Scholar] [CrossRef]
- Duan, Z.Q.; Du, W.; Liu, D.H. The mechanism of solvent effect on the positional selectivity of Candida antarctica lipase B during 1,3-diolein synthesis by esterification. Bioresour. Technol. 2011, 102, 11048–11050. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.F.; Fornari, T.; Torrelo, G.; Señoráns, F.J.; Reglero, G. Production of phytosterol esters from soybean oil deodorizer distillates. Eur. J. Lipid Sci. Technol. 2009, 111, 459–463. [Google Scholar] [CrossRef]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P. Lipases in lipophilization reactions. Biotechnol. Adv. 2007, 25, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Napier, P.E.; Lacerda, H.M.; Rosell, C.M.; Valivety, R.H.; Vaidya, A.M.; Halling, P.J. Enhanced organic-phase enzymatic esterification with continuous water removal in a controlled air-bleed evacuated-headspace reactor. Biotechnol. Prog. 1996, 12, 47–50. [Google Scholar] [CrossRef]
- Meissner, J.P.; Carta, G. Continuous Regioselective Enzymatic Esterification in a Simulated Moving Bed Reactor. Ind. Eng. Chem. Res. 2002, 41, 4722–4732. [Google Scholar] [CrossRef]
- Gubicza, L.; Nemestóthy, N.; Fráter, T.; Bélafi-Bakó, K. Enzymatic esterification in ionic liquids integrated with pervaporation for water removal. Green Chem. 2003, 5, 236–239. [Google Scholar] [CrossRef]
- Bloomer, S.; Adlercreutz, P.; Mattiasson, B. Facile synthesis of fatty acid esters in high yields. Enzyme Microb. Technol. 1992, 14, 546–552. [Google Scholar] [CrossRef]
- Jeong, J.C.; Lee, S.B. Enzymatic esterification reaction in organic media with continuous water stripping: Effect of water content on reactor performance and enzyme agglomeration. Biotechnol. Technol. 1997, 11, 853–858. [Google Scholar] [CrossRef]
- Won, K.; Lee, S.B. Online conversion estimation for solvent-free enzymatic esterification systems with water activity control. Biotechnol. Bioprocess Eng. 2002, 7, 76–84. [Google Scholar] [CrossRef]
- Won, K.; Lee, S.B. Computer-aided control of water activity for lipase-catalyzed esterification in solvent-free systems. Biotechnol. Prog. 2001, 17, 258–264. [Google Scholar] [CrossRef]
- Petersson, A.E.V.; Adlercreutz, P.; Mattiasson, B. A water activity control system for enzymatic reactions in organic media. Biotechnol. Bioeng. 2007, 97, 235–241. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Bansode, S.R.; Rathod, V.K. Ultrasound assisted lipase catalysed synthesis of isoamyl butyrate. Process Biochem. 2014, 49, 1297–1303. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, J.H.; Chang, C.; Shieh, C.J. Optimization of ultrasound-accelerated synthesis of enzymatic caffeic acid phenethyl ester by response surface methodology. Ultrason. Sonochem. 2011, 18, 455–459. [Google Scholar] [CrossRef]
- Réjasse, B.; Lamare, S.; Legoy, M.D.; Besson, T. Stability improvement of immobilized Candida antarctica lipase B in an organic medium under microwave radiation. Org. Biomol. Chem. 2004, 2, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.; Zabot, G.L.; Meireles, M.A.A.; Mazutti, M.A.; Martínez, J. Synthesis of eugenyl acetate by enzymatic reactions in supercritical carbon dioxide. Biochem. Eng. J. 2016, 114, 1–9. [Google Scholar] [CrossRef]
- Nyström, L. Analysis Methods of Phytosterols. Anal. Antioxid.-Rich Phytochem. 2012, 313–351. [Google Scholar] [CrossRef]
- Piironen, V.; Lampi, A.-M. Occurrence and Levels of Phytosterols in Foods. In Phytosterols as Functional Food Components and Nutraceuticals; Dutta, P.C., Ed.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Abe, H.; Soeno, K.; Koseki, N.N.; Natsume, M. Conjugated and unconjugated brassinosteroids. In ACS Symposium Series; ACS: Washington, DC, USA, 2001; Volume 774, pp. 91–101. [Google Scholar] [CrossRef]
- Carretero, A.S.; Carrasco-Pancorbo, A.; Cortacero, S.; Gori, A.; Cerretani, L.; Fernández-Gutiérrez, A. A simplified method for HPLC-MS analysis of sterols in vegetable oil. Eur. J. Lipid Sci. Technol. 2008, 110, 1142–1149. [Google Scholar] [CrossRef]
- Breinhölder, P.; Mosca, L.; Lindner, W. Concept of sequential analysis of free and conjugated phytosterols in different plant matrices. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 67–82. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, Z.; Liu, R.; Zhang, H.; Zhu, H.; Jiang, L.; Tsao, R. A comprehensive profiling of free, conjugated and bound phenolics and lipophilic antioxidants in red and green lentil processing by-products. Food Chem. 2020, 325, 126925. [Google Scholar] [CrossRef]
- Junker, J.; Chong, I.; Kamp, F.; Steiner, H.; Giera, M.; Müller, C.; Bracher, F. Comparison of strategies for the determination of sterol sulfates via GC-MS leading to a novel deconjugation-derivatization protocol. Molecules 2019, 24, 2353. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; McEwan, M.J.; Španěl, P. Understanding Gas Phase Ion Chemistry Is the Key to Reliable Selected Ion Flow Tube-Mass Spectrometry Analyses. Anal. Chem. 2020, 92, 12750–12762. [Google Scholar] [CrossRef]
- Ishida, N. A method for simultaneous analysis of phytosterols and phytosterol esters in tobacco leaves using non aqueous reversed phase chromatography and atmospheric pressure chemical ionization mass spectrometry detector. J. Chromatogr. A 2014, 1340, 99–108. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Tao, G.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Characterization and determination of free phytosterols and phytosterol conjugates: The potential phytochemicals to classify different rice bran oil and rice bran. Food Chem. 2021, 344, 128624. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Caprioli, G.; Ricciutelli, M.; Cortese, M.; Alesi, A.; Vittori, S.; Sagratini, G. Development of an innovative phytosterol derivatization method to improve the HPLC-DAD analysis and the ESI-MS detection of plant sterols/stanols. Food Res. Int. 2020, 131, 108998. [Google Scholar] [CrossRef]
- de Sousa, R.R.; da Silva, A.S.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Simplified method to optimize enzymatic esters syntheses in solvent-free systems: Validation using literature and experimental data. Catalysts 2021, 11, 1357. [Google Scholar] [CrossRef]




| Product | Enzyme | Solvent | Temperature (°C) | Time (h) | Conversion (%) | Ref. |
|---|---|---|---|---|---|---|
| β-sitosterol laurate | Candida rugosa lipase | Isooctane | 47 | 48 | 88.12 ± 0.79 | [73] |
| Functional oil | Candida rugosa and Thermomyces lanuginosus lipase | n-heptane | 40 | 8 | - | [74] |
| Stigmasteryl oleate | Candida rugosa lipase | Petroleum ether | 45 | 16 | 97.33 | [75] |
| Rice bran oil | Novozym 435 | Solvent-Free | 78 | 41 | 93.2 | [76] |
| β-sitosterol linolenate | Candida rugosa lipase | Isooctane | 29.5 | 6 | 96.8 ± 0.7 | [77] |
| Phytosterols linolenate | Candida rugosa lipase | Solvent-Free | 50 | 1.17 | 90.0 ± 2 | [78] |
| Rice soybean oil | Candida antarctica lipase B | Solvent-Free | 50 | 3 | 86.2 | [79] |
| Phytosterol ester | Candida rugosa lipase | Cyclohexane | 30 | 8 | 81.0 | [80] |
| Phytosterol oleate | Candida rugosa lipase | Isooctane | 50 | 6 | 96.5 | [81] |
| Phytosterol oleate | Yarrowia lipolytica lipase | Solvent-free | 50 | 72 | 91.1 | [82] |
| Phytosterol ester | Candida rugosa lipase | n-heptane | 44 | 12 | 90.8 ± 0.4 | [83] |
| Phytosterol Linolenate | Candida rugosa lipase | Isooctane | 40 | 2 | 95.9 ± 0.8 | [65] |
| Phytosteryl Ester | Candida rugosa lipase | n-hexane | 40 | 0.05 | 90.0 | [84] |
| Phytosteryl laurate | Candida rugosa lipase | n-hexane | 50 | 48 | 95.1 | [85] |
| Phytosterol oleate | Candida rugosa lipase | Isooctane | 50 | 1 | 95.4 | [86] |
| Functional Oil | Candida rugosa lipase | Hexane | 50 | 12 | >92.1 | [87] |
| Phytosterol oleate | Candida rugosa lipase | - | - | 0.6 | 75.26 | [88] |
| Phytosterol oleate | Candida rugosa lipase | - | 30 | 24 | 80.0 | [89] |
| Phytosteryl ester | Candida rugosa lipase | Solvent-free | 60 | 1.5 | 93.0 | [90] |
| β-sitosteryl esters | Candida antarctica lipase | Hexane | 40–50 | 24 | 93.0–98.0 | [91] |
| Phytosterol esters | Candida rugosa lipase | Isooctane | 50 | 7 | >92.1 | [92] |
| Phytosterol laurate | Candida rugosa lipase | n-hexane | 40 | 10 | 96.6 | [93] |
| Phytosterol oleate | Candida sp. lipase | Isooctane | 45 | 24 | 93.4 | [94] |
| Phytosterol esters | Candida rugosa lipase | Isooctane | 40–55 | 6–24 | >80.0 | [95] |
| Phytosteryl esters | Candida rugosa and Pseudomonas stutzeri lipase | Solvent-free | 50–60 | 3–4 | >90.0 | [96] |
| Phytosteryl Caprylates | Candida rugosa lipase | n-hexane | 45 | 9 | 98.0 | [97] |
| Phytosterols linolenate | Candida rugosa lipase | Isooctane | 55 | 15 | 93.5 | [98] |
| Phytosteryl esters | Pseudomonas stutzeri lipase | Free and exogenous solvent | 50 | - | - | [99] |
| Phytosteryl esters | Different enzymes | Solvent-free | 25 and 50 | 4–312 | 5.0–97.0 | [100] |
| β-sitosterol esters | Thermomyces lanuginosus lipase | - | 40–65 | 3–24 | - | [101] |
| Phytosteryl linolenate | Candida rugosa lipase | Solvent-free | 35–40 | - | <80 | [102] |
| Phytosteryl esters | Candida rugosa lipase | Solvent-free | 50 | 9 and 48 | 94.0–99.0 | [103] |
| Phytosterol oleate | Candida rugosa lipase | n-hexane | 51 | 17 | 97.0 | [104] |
| Phytosterol oleate | Different enzymes | n-hexane | 35 | 24–72 | <85.0 | [105] |
| Sitosteryl esters | Different enzymes | With or without solvent | 55 | 48 | <30.0 | [106] |
| Phytosterol ester | Alcaligenes sp. lipase | Solvent-free | 100 | 7 | 97.1 | [107] |
| Phytosteryl linolenate | Rhizomucor miehei lipase | - | 50 | 24 | - | [108] |
| Phytosterol ester | Candida rugosa lipase | Solvent-free | 35 | 5 | 90.0 | [109] |
| Phytosterol ester | Candida rugosa lipase | isooctane | 55 | - | 93.6 | [110] |
| Phytosterol ester | Proteus vulgaris K80 lipase | hexane | 40 | 3 | 71.0 | [111] |
| Phytosterol ester | Novozyme 435 lipase | - | 101 | 3 | 85.6 | [112] |
| Phytosterol laurate | - | - | 45 | 48 | 94.6 | [113] |
| Ergosterol linolenate | Candida sp. 99–125 lipase | isooctane | 45 | 12 | 92.0 | [114] |
| Phytosterol ester | Candida rugosa lipase | Solvent-free | 55 | 1.5 | 95.0 | [115] |
| Phytosterol ester | Lipase AYS | Isooctane | 45 | 0.16 | 90.0 | [116] |
| Functional oil | Candida rugosa lipase | Isooctane | 55 | 2 | 85.0 | [117] |
| Phytosterol ester | Candida rugosa lipase | Isooctane | 50 | 2 | 96.8 | [118] |
| Phytosterol ester | Lipozyme 435 lipase | n-hexane | 55 | 20 | 93.0 | [119] |
| Phytosteryl docosahexaenoates | Pseudomonas sp. lipase | n-hexane | - | 24 | 96.0 | [120] |
| Phytosteryl lipoate | Candida rugosa lipase | 2 -metil-2-butanol/n-hexano | 55 | 96 | 71.2 | [121] |
| Steryl hydroxycinnamates | Candida rugosa lipase | n-hexane | 63 | 120 | 55 | [122] |
| Functional oil | Candida antarctica lipase | - | - | - | 87.4 | [123] |
| Phytosterol ester | Candida rugosa lipase | n-hexane | 45 | 48 | 84.7 | [124] |
| β-sitosterol linolenate | - | n-hexane | 50 | 72 | 72.6 | [125] |
| Phytosterol ester | Candida rugosa lipase | Solvent-free | 55 | - | 90.0 | [126] |
| Linoleyl β-sitosterol | Candida antarctica lipase | n-hexane | 50 | 72 | 72.6 | [127] |
| Phytosterol myristate | Lipase AYS | n-hexane | 50 | 72 | 69.9 | [128] |
| Phytosterol palmitate | Novozyme 435 lipase | n-hexane | 55 | 72 | 36.9 | [129] |
| Functional oil | Novozyme 435 lipase | Solvent-free | 85 | 1 | 92.0 | [130] |
| Phytosterol ester | Pseudomonas lipase | - | 55.5 | 41.2 | 84.4 | [131] |
| Phytosterol ester | Candida rugosa lipase | n-hexane | 40 | 87 | 97.5 | [132] |
| Phytosterol linolenate | Novozyme 435 lipase | Isooctane | 55 | 24 | 40.6 | [133] |
| Phytosterol ester | Candida antarctica lipase | - | 50 | 72 | 85.7 | [134] |
| Phytosterol laurate | - | Solvent-free | 60 | 12 | 92.2 | [135] |
| Functional oil | Thermomyces lanuginosus lipase | n-hexane | 60 | 25 | - | [136] |
| Phytosterol ester | Canadia sp. 99–125 lipase | Isooctane | 60 | 8 | 85.7 | [137] |
| Title | Inventors | Registration Number | Registration Date |
|---|---|---|---|
| Method for preparing functional edible oil rich in phytosterol esters and diglycerides | [140] | US20150289534A1 | 15 October 2015 |
| Method for producing fatty acid phytosterol ester using immobilized lipase derived from Candida rugosa | [141] | KR101550101B1 KR20150046397A | 30 April 2015 4 September 2015 |
| Method for producing phytosterol/phytostanol phospholipid esters | [142] | WO2010109441A1 | 30 September 2010 |
| Method for producing plant sterol ester by immobilized whole-cell enzyme catalysis in solvent-free system | [143] | CN101200754A | 18 June 2008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.d.S.; de Souza, A.H.; Fraga, J.L.; Villeneuve, P.; Torres, A.G.; Amaral, P.F.F. Lipases as Effective Green Biocatalysts for Phytosterol Esters’ Production: A Review. Catalysts 2022, 12, 88. https://doi.org/10.3390/catal12010088
Pereira AdS, de Souza AH, Fraga JL, Villeneuve P, Torres AG, Amaral PFF. Lipases as Effective Green Biocatalysts for Phytosterol Esters’ Production: A Review. Catalysts. 2022; 12(1):88. https://doi.org/10.3390/catal12010088
Chicago/Turabian StylePereira, Adejanildo da S., Aline Habibe de Souza, Jully L. Fraga, Pierre Villeneuve, Alexandre G. Torres, and Priscilla F. F. Amaral. 2022. "Lipases as Effective Green Biocatalysts for Phytosterol Esters’ Production: A Review" Catalysts 12, no. 1: 88. https://doi.org/10.3390/catal12010088
APA StylePereira, A. d. S., de Souza, A. H., Fraga, J. L., Villeneuve, P., Torres, A. G., & Amaral, P. F. F. (2022). Lipases as Effective Green Biocatalysts for Phytosterol Esters’ Production: A Review. Catalysts, 12(1), 88. https://doi.org/10.3390/catal12010088