Hierarchical Porous Carbon Fibers for Enhanced Interfacial Electron Transfer of Electroactive Biofilm Electrode
Abstract
:1. Introduction
2. Results
2.1. Characterization of NPCFs
2.2. Bioelectrocatalysis Behavior Analyses
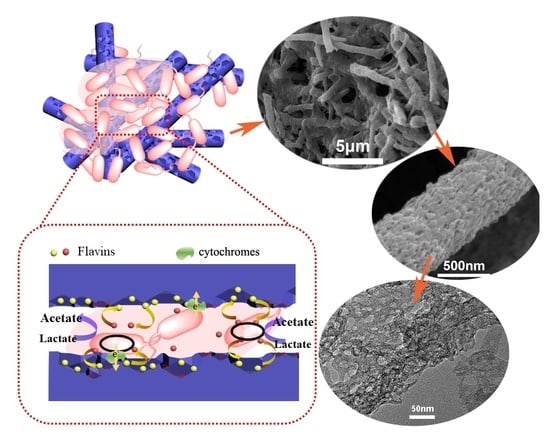
2.3. Biofilm Observation
2.4. Nanoporous Fiber Dependent Interfacial Electron Transfer Mechanism
3. Materials and Methods
3.1. Preparation of ZIF-8 Particles
3.2. Preparation of Mesoporous Carbon Fibers
3.3. Bacterial Culture
3.4. Material Characterization
3.5. MFC Set Up and Operation
3.6. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, A.; Hsu, L.H.-H.; Kavanagh, P.; Barrière, F.; Lens, P.N.L.; Lapinsonnière, L.; Lienhard V, J.H.; Schröder, U.; Jiang, X.; Leech, D. The ins and outs of microorganism–electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1, 0024. [Google Scholar] [CrossRef] [Green Version]
- Brutinel, E.D.; Gralnick, J.A. Shuttling happens: Soluble flavin mediators of extracellular electron transfer in Shewanella. Appl. Microbiol. Biotechnol. 2011, 93, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Qiao, Y.; Wu, Z.-Y.; Wu, X.-S.; Xie, J.-L.; Yu, S.-H.; Guo, J.; Li, C.M. Tailoring Unique Mesopores of Hierarchically Porous Structures for Fast Direct Electrochemistry in Microbial Fuel Cells. Adv. Energy Mater. 2015, 6, 1501535. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.-S.; Qiao, Y.; Wang, R.-J.; Luo, X. Tailoring of pore structure in mesoporous carbon for favourable flavin mediated interfacial electron transfer in microbial fuel cells. RSC Adv. 2018, 8, 9597–9602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Qiao, Y.-J.; Zou, L.; Ma, C.-X.; Liu, J.-H. Real-time monitoring of phenazines excretion in Pseudomonas aeruginosa microbial fuel cell anode using cavity microelectrodes. Bioresour. Technol. 2015, 198, 1–6. [Google Scholar] [CrossRef]
- Qiao, Y.-J.; Qiao, Y.; Zou, L.; Wu, X.-S.; Liu, J.-H. Biofilm promoted current generation of Pseudomonas aeruginosa microbial fuel cell via improving the interfacial redox reaction of phenazines. Bioelectrochemistry 2017, 117, 34–39. [Google Scholar] [CrossRef]
- Yong, Y.-C.; Dong, X.-C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and Monolithic Anode Based on Polyaniline Hybridized Three-Dimensional Graphene for High-Performance Microbial Fuel Cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.-C.; Yu, Y.-Y.; Zhang, X.; Song, H. Highly Active Bidirectional Electron Transfer by a Self-Assembled Electroactive Reduced-Graphene-Oxide-Hybridized Biofilm. Angew. Chem. Int. Ed. 2014, 53, 4480–4483. [Google Scholar] [CrossRef]
- Zou, L.; Huang, Y.; Wu, X.; Long, Z.-E. Synergistically promoting microbial biofilm growth and interfacial bioelectrocatalysis by molybdenum carbide nanoparticles functionalized graphene anode for bioelectricity production. J. Power Sources 2018, 413, 174–181. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Li, Y.; Bao, W.; Fang, Z.; Preston, C.; Vaaland, O.; Ren, Z.; Hu, L. Lightweight, conductive hollow fibers from nature as sustainable electrode materials for microbial energy harvesting. Nano Energy 2014, 10, 268–276. [Google Scholar] [CrossRef]
- Singh, S.; Bairagi, P.K.; Verma, N. Candle soot-derived carbon nanoparticles: An inexpensive and efficient electrode for microbial fuel cells. Electrochim. Acta 2018, 264, 119–127. [Google Scholar] [CrossRef]
- Wu, X.; Qian, Y.; Shi, Z.; Li, C. Enhancement of interfacial bioelectrocatalysis in Shewanella microbial fuel cells by a hierarchical porous carbon–silica composite derived from distiller’s grains. Sustain. Energ. Fuels 2018, 2, 655–662. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.-Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the Challenges of Enzymatic (Bio)Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef]
- Zou, L.; Qiao, Y.; Zhong, C.; Li, C.M. Enabling fast electron transfer through both bacterial outer-membrane redox centers and endogenous electron mediators by polyaniline hybridized large-mesoporous carbon anode for high-performance microbial fuel cells. Electrochim. Acta 2017, 229, 31–38. [Google Scholar] [CrossRef]
- Choi, S.; Kim, B.; Chang, I.S. Tracking of Shewanella oneidensis MR-1 biofilm formation of a microbial electrochemical system via differential pulse voltammetry. Bioresour. Technol. 2018, 254, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.H.; Kim, Y.S.; Oh, S.-E. Power generation from cellulose using mixed and pure cultures of cellulose-degrading bacteria in a microbial fuel cell. Enzym. Microb. Technol. 2012, 51, 269–273. [Google Scholar] [CrossRef]
- Manickam, S.S.; Karra, U.; Huang, L.; Bui, N.-N.; Li, B.; McCutcheon, J.R. Activated carbon nanofiber anodes for microbial fuel cells. Carbon 2013, 53, 19–28. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Huang, H.; Mai, Y.W.; Zhou, L. Electrospun carbon-based nanostructured electrodes for advanced energy storage—A review. Energy Storage Mater. 2016, 5, 58–92. [Google Scholar] [CrossRef]
- Chen, S.; Hou, H.; Harnisch, F.; Patil, S.A.; Carmona-Martinez, A.A.; Agarwal, S.; Zhang, Y.; Sinha-Ray, S.; Yarin, A.L.; Greiner, A.; et al. Electrospun and solution blown three-dimensional carbon fiber nonwovens for application as electrodes in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1417–1421. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun Nanomaterials for Supercapacitor Electrodes: Designed Architectures and Electrochemical Performance. Adv. Energy Mater. 2016, 7. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Jung, J.-Y.; Jung, M.-J.; Lee, Y.-S. Hierarchical porous carbon fibers prepared using a SiO2 template for high-performance EDLCs. Chem. Eng. J. 2015, 263, 62–70. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun Fibrous Mats with High Porosity as Potential Scaffolds for Skin Tissue Engineering. Biomacromolecules 2008, 9, 1795–1801. [Google Scholar] [CrossRef]
- Feng, C.; Lv, Z.; Yang, X.; Wei, C. Anode modification with capacitive materials for a microbial fuel cell: An increase in transient power or stationary power. Phys. Chem. Chem. Phys. 2014, 16, 10464–10472. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Gu, Y.; He, S.; Schroder, U.; Chen, S.; Hou, H. Effect of fiber diameter on the behavior of biofilm and anodic performance of fiber electrodes in microbial fuel cells. Bioresour. Technol. 2011, 102, 10763–10766. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.; Erable, B.; Bergel, A. Effect of pore size on the current produced by 3-dimensional porous microbial anodes: A critical review. Bioresour. Technol. 2019, 289, 121641. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.M.; Ulstrup, J.; Zhao, F. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 2017, 3, e1700623. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, H.; Huang, L.; Li, X.; Yu, L.; Zeng, S.; Zeng, X.; Yang, J.; Zou, J. Nitrogen-enriched hierarchically porous carbon nanofiber network as a binder-free electrode for high-performance supercapacitors. Electrochim. Acta 2017, 246, 606–614. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, X.-S.; Li, C.M. Interfacial electron transfer of Shewanella putrefaciens enhanced by nanoflaky nickel oxide array in microbial fuel cells. J. Power Sources 2014, 266, 226–231. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wu, X.; Liu, C.; Yang, J.; Luo, X.; Zou, L.; Lu, Z.; Qiao, Y. Hierarchical Porous Carbon Fibers for Enhanced Interfacial Electron Transfer of Electroactive Biofilm Electrode. Catalysts 2022, 12, 1187. https://doi.org/10.3390/catal12101187
Wang R, Wu X, Liu C, Yang J, Luo X, Zou L, Lu Z, Qiao Y. Hierarchical Porous Carbon Fibers for Enhanced Interfacial Electron Transfer of Electroactive Biofilm Electrode. Catalysts. 2022; 12(10):1187. https://doi.org/10.3390/catal12101187
Chicago/Turabian StyleWang, Ruijie, Xiaoshuai Wu, Chang Liu, Jing Yang, Xian Luo, Long Zou, Zhisong Lu, and Yan Qiao. 2022. "Hierarchical Porous Carbon Fibers for Enhanced Interfacial Electron Transfer of Electroactive Biofilm Electrode" Catalysts 12, no. 10: 1187. https://doi.org/10.3390/catal12101187
APA StyleWang, R., Wu, X., Liu, C., Yang, J., Luo, X., Zou, L., Lu, Z., & Qiao, Y. (2022). Hierarchical Porous Carbon Fibers for Enhanced Interfacial Electron Transfer of Electroactive Biofilm Electrode. Catalysts, 12(10), 1187. https://doi.org/10.3390/catal12101187