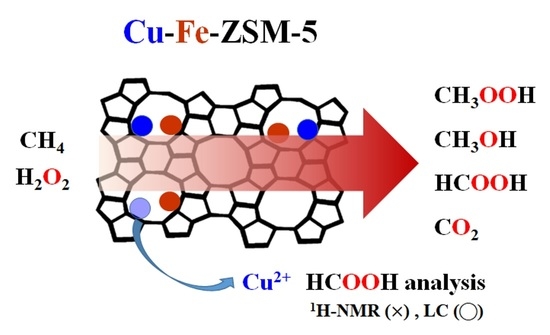
Effects of Cu Species on Liquid-Phase Partial Oxidation of Methane with H2O2 over Cu-Fe/ZSM-5 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of the Prepared Catalysts
2.2. Catalytic Activity in a Batch Reactor
2.3. Catalytic Activity in a Flow Reactor
3. Experiment
3.1. Catalyst Preparation
3.1.1. Solid-State Ion-Exchange (SIE) Method
3.1.2. Wet Impregnation (WI) Method
3.1.3. Ion-Exchange (IE) Method
3.2. Activity Test
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, K.; Kang, J.; King, D.L.; Subramanian, V.; Zhou, C.; Zhang, Q.; Wang, Y. Chapter Three—Advances in Catalysis for Syngas Conversion to Hydrocarbons. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 60, pp. 125–208. [Google Scholar]
- Siirola, J.J. The impact of shale gas in the chemical industry. AIChE J. 2014, 60, 810–819. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, Q.; Wu, Z.; Ma, D. Methane activation: The past and future. Energy Environ. Sci. 2014, 7, 2580–2591. [Google Scholar] [CrossRef]
- Olivos-Suarez, A.I.; Szécsényi, À.; Hensen, E.J.M.; Ruiz-Martinez, J.; Pidko, E.A.; Gascon, J. Strategies for the Direct Catalytic Valorization of Methane Using Heterogeneous Catalysis: Challenges and Opportunities. ACS Catal. 2016, 6, 2965–2981. [Google Scholar] [CrossRef]
- Schwach, P.; Pan, X.; Bao, X. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Guan, N.; Li, L. Methane Activation and Utilization: Current Status and Future Challenges. Energy Technol. 2020, 8, 1900826. [Google Scholar] [CrossRef]
- Ravi, M.; Ranocchiari, M.; van Bokhoven, J.A. The Direct Catalytic Oxidation of Methane to Methanol—A Critical Assessment. Angew. Chem. Int. Ed. 2017, 56, 16464–16483. [Google Scholar] [CrossRef]
- Taifan, W.; Baltrusaitis, J. CH4 conversion to value added products: Potential, limitations and extensions of a single step heterogeneous catalysis. Appl. Catal. B 2016, 198, 525–547. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Peppel, T.; Seeburg, D.; Kondratenko, V.A.; Kalevaru, N.; Martin, A.; Wohlrab, S. Methane conversion into different hydrocarbons or oxygenates: Current status and future perspectives in catalyst development and reactor operation. Catal. Sci. Technol. 2017, 7, 366–381. [Google Scholar] [CrossRef]
- Hammond, C.; Conrad, S.; Hermans, I. Oxidative Methane Upgrading. ChemSusChem 2012, 5, 1668–1686. [Google Scholar] [CrossRef]
- Xu, Z.C.; Park, E.D. Gas-Phase Selective Oxidation of Methane into Methane Oxygenates. Catalysts 2022, 12, 314. [Google Scholar] [CrossRef]
- Gunsalus, N.J.; Koppaka, A.; Park, S.H.; Bischof, S.M.; Hashiguchi, B.G.; Periana, R.A. Homogeneous Functionalization of Methane. Chem. Rev. 2017, 117, 8521–8573. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Soorholtz, M.; Bilke, M.; Schüth, F. Selective Methane Oxidation Catalyzed by Platinum Salts in Oleum at Turnover Frequencies of Large-Scale Industrial Processes. J. Ame. Chem. Soc. 2016, 138, 12395–12400. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.; Lee, H.W.; Lee, J.; Choo, H.; Hong, S.H.; Cheong, M.; Lee, H. Enhanced Catalytic Activity of (DMSO)2PtCl2 for the Methane Oxidation in the SO3–H2SO4 System. ACS Catal. 2018, 8, 11854–11862. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Han, Z.; Huang, S.; Yuan, D.; Su, W. Atmosphere-Pressure Methane Oxidation to Methyl Trifluoroacetate Enabled by a Porous Organic Polymer-Supported Single-Site Palladium Catalyst. ACS Catal. 2021, 11, 1008–1013. [Google Scholar] [CrossRef]
- Zimmermann, T.; Bilke, M.; Soorholtz, M.; Schüth, F. Influence of Catalyst Concentration on Activity and Selectivity in Selective Methane Oxidation with Platinum Compounds in Sulfuric Acid and Oleum. ACS Catal. 2018, 8, 9262–9268. [Google Scholar] [CrossRef]
- Hashiguchi, B.G.; Bischof, S.M.; Konnick, M.M.; Periana, R.A. Designing Catalysts for Functionalization of Unactivated C–H Bonds Based on the CH Activation Reaction. Acc. Chem. Res. 2012, 45, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Dang, H.T.; Kim, H.; Lee, U.; Ha, J.-M.; Jae, J.; Cheong, M.; Lee, H. Pt black catalyzed methane oxidation to methyl bisulfate in H2SO4-SO3. J. Catal. 2019, 374, 230–236. [Google Scholar] [CrossRef]
- Ravi, M.; Bokhoven, J. Homogeneous Copper-Catalyzed Conversion of Methane to Methyl Trifluoroacetate in High Yield at Low Pressure. ChemCatChem 2018, 10, 2383–2386. [Google Scholar] [CrossRef]
- Blankenship, A.N.; Ravi, M.; Newton, M.A.; van Bokhoven, J.A. Heterogeneously Catalyzed Aerobic Oxidation of Methane to a Methyl Derivative. Angew. Chem. Int. Ed. 2021, 60, 18138–18143. [Google Scholar] [CrossRef]
- Freakley, S.J.; Dimitratos, N.; Willock, D.J.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Methane Oxidation to Methanol in Water. Acc. Chem. Res. 2021, 54, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Maji, S.; Chen, P.; Lee, H.; Yu, S.; Chan, S. Alkane Oxidation: Methane Monooxygenases, Related Enzymes, and Their Biomimetics. Chem. Rev. 2017, 117, 8574–8621. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.E.R.; Bols, M.L.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. Iron and Copper Active Sites in Zeolites and Their Correlation to Metalloenzymes. Chem. Rev. 2018, 118, 2718–2768. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.W.C.E.; Lempers, H.E.B. Liquid phase oxidation at metal ions and complexes in constrained environments. Catal. Today 1998, 41, 387–407. [Google Scholar] [CrossRef]
- Sarkar, S.; Moser, M.L.; Tian, X.; Zhang, X.; Al-Hadeethi, Y.F.; Haddon, R.C. Metals on Graphene and Carbon Nanotube Surfaces: From Mobile Atoms to Atomtronics to Bulk Metals to Clusters and Catalysts. Chem. Mater. 2014, 26, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal–Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, J.; Hirayama, A.; Kondou, N.; Yoshida, H.; Machida, M.; Nishimura, S.; Hirai, K.; Miyazato, I.; Takahashi, K. Data science assisted investigation of catalytically active copper hydrate in zeolites for direct oxidation of methane to methanol using H2O2. Sci. Rep. 2021, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Szécsényi, Á.; Li, G.; Gascon, J.; Pidko, E.A. Mechanistic Complexity of Methane Oxidation with H2O2 by Single-Site Fe/ZSM-5 Catalyst. ACS Catal. 2018, 8, 7961–7972. [Google Scholar] [CrossRef]
- He, Y.; Liang, J.; Imai, Y.; Ueda, K.; Li, H.; Guo, X.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Highly selective synthesis of methanol from methane over carbon materials supported Pd-Au nanoparticles under mild conditions. Catal. Today 2020, 352, 104–110. [Google Scholar] [CrossRef]
- Hong, S.; Mpourmpakis, G. Mechanistic understanding of methane-to-methanol conversion on graphene-stabilized single-atom iron centers. Catal. Sci. Technol. 2021, 11, 6390–6400. [Google Scholar] [CrossRef]
- Cui, X.; Li, H.; Wang, Y.; Hu, Y.; Hua, L.; Li, H.; Han, X.; Liu, Q.; Yang, F.; He, L.; et al. Room-Temperature Methane Conversion by Graphene-Confined Single Iron Atoms. Chem 2018, 4, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Tahini, H.A.; Smith, S.C. Defect Engineering in Graphene-Confined Single-Atom Iron Catalysts for Room-Temperature Methane Conversion. J. Phys. Chem. C 2021, 125, 12628–12635. [Google Scholar] [CrossRef]
- Kholdeeva, O.; Maksimchuk, N. Metal-Organic Frameworks in Oxidation Catalysis with Hydrogen Peroxide. Catalysts 2021, 11, 283. [Google Scholar] [CrossRef]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Kianfar, E.; Hajimirzaee, S.; mousavian, S.; Mehr, A.S. Zeolite-based catalysts for methanol to gasoline process: A review. Microchem. J. 2020, 156, 104822. [Google Scholar] [CrossRef]
- Kalck, P.; Le Berre, C.; Serp, P. Recent advances in the methanol carbonylation reaction into acetic acid. Coord. Chem. Rev 2020, 402, 213078. [Google Scholar] [CrossRef]
- Hammond, C.; Forde, M.M.; Ab Rahim, M.H.; Thetford, A.; He, Q.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Dummer, N.F.; Murphy, D.M.; et al. Direct Catalytic Conversion of Methane to Methanol in an Aqueous Medium by using Copper-Promoted Fe-ZSM-5. Angew. Chem. Int. Ed. 2012, 51, 5129–5133. [Google Scholar] [CrossRef]
- Al-Shihri, S.; Richard, C.J.; Al-Megren, H.; Chadwick, D. Insights into the direct selective oxidation of methane to methanol over ZSM-5 zeolytes in aqueous hydrogen peroxide. Catal. Today 2020, 353, 269–278. [Google Scholar] [CrossRef]
- Yu, T.; Li, Z.; Lin, L.; Chu, S.; Su, Y.; Song, W.; Wang, A.; Weckhuysen, B.M.; Luo, W. Highly Selective Oxidation of Methane into Methanol over Cu-Promoted Monomeric Fe/ZSM-5. ACS Catal. 2021, 11, 6684–6691. [Google Scholar] [CrossRef]
- Forde, M.M.; Armstrong, R.D.; McVicker, R.; Wells, P.P.; Dimitratos, N.; He, Q.; Lu, L.; Jenkins, R.L.; Hammond, C.; Lopez-Sanchez, J.A.; et al. Light alkane oxidation using catalysts prepared by chemical vapour impregnation: Tuning alcohol selectivity through catalyst pre-treatment. Chem. Sci. 2014, 5, 3603–3616. [Google Scholar] [CrossRef]
- Forde, M.M.; Armstrong, R.D.; Hammond, C.; He, Q.; Jenkins, R.L.; Kondrat, S.A.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Willock, D.; et al. Partial Oxidation of Ethane to Oxygenates Using Fe- and Cu-Containing ZSM-5. J. Ame. Chem. Soc. 2013, 135, 11087–11099. [Google Scholar] [CrossRef] [PubMed]
- Praliaud, H.; Mikhailenko, S.D.; Chajar, Z.; Primet, M. Surface and bulk properties of Cu- ZSM-5 and Cu/Al2O3 solids during redox treatments. Correlation with the selective reduction of nitric oxide by hydrocarbons. Appl. Catal. B 1998, 16, 359–374. [Google Scholar] [CrossRef]
- Wichterlová, B.; Dědeček, J.; Sobalík, Z.; Vondrová, A.; Klier, K. On the Cu Site in ZSM-5 Active in Decomposition of NO: Luminescence, FTIR Study, and Redox Properties. J. Catal. 1997, 169, 194–202. [Google Scholar] [CrossRef]
- Jouini, H.; Mejri, I.; Petitto, C.; Martinez-Ortigosa, J.; Vidal-Moya, A.; Mhamdi, M.; Blasco, T.; Delahay, G. Characterization and NH3-SCR reactivity of Cu-Fe-ZSM-5 catalysts prepared by solid state ion exchange: The metal exchange order effect. Microporous Mesoporous Mater. 2018, 260, 217–226. [Google Scholar] [CrossRef]
- Heinrich, F.; Schmidt, C.; Löffler, E.; Menzel, M.; Grünert, W. Fe–ZSM-5 Catalysts for the Selective Reduction of NO by Isobutane—The Problem of the Active Sites. J. Catal. 2002, 212, 157–172. [Google Scholar] [CrossRef]
- Delahay, G.; Guzman-Vargas, A.; Valade, D.; Coq, B. Selective Catalytic Reduction of no by NH3 on Fe-ZSM-5 Elaborated from Different Methods. Stud. Surf. Sci. Catal. 2004, 154 C, 2501–2508. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, E.D. Aqueous-phase partial oxidation of methane with H2O2 over Fe-ZSM-5 catalysts prepared from different iron precursors. Microporous Mesoporous Mater. 2021, 324, 111278. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, K.H.; Cho, S.J.; Park, E.D. Partial oxidation of methane with hydrogen peroxide over Fe-ZSM-5 catalyst. Catal. Today 2021, 376, 113–118. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Saussey, J.; Freysz, J.L.; Lavalley, J.C. FT-IR study of NO + O2 co-adsorption on H-ZSM-5: Re-assignment of the 2133 cm−1 band to NO+ species. Catal. Lett. 1998, 52, 103–108. [Google Scholar] [CrossRef]
- Lobree, L.J.; Hwang, I.C.; Reimer, J.A.; Bell, A.T. Investigations of the State of Fe in H ZSM-5. J. Catal. 1999, 186, 242–253. [Google Scholar] [CrossRef]
- Lezcano, M.; Kovalchuk, V.I.; D’itri, J.L. FTIR Study of the Interaction of Nitric Oxide with Fe-ZSM-51. Kinet. Catal. 2001, 42, 104–111. [Google Scholar] [CrossRef]
- Mul, G.; Pérez-Ramírez, J.; Kapteijn, F.; Moulijn, J.A. NO Adsorption on Ex-Framework [Fe,X]MFI Catalysts: Novel IR Bands and Evaluation of Assignments. Catal. Lett. 2002, 80, 129–138. [Google Scholar] [CrossRef]
- Giordanino, F.; Vennestrøm, P.N.R.; Lundegaard, L.F.; Stappen, F.N.; Mossin, S.; Beato, P.; Bordiga, S.; Lamberti, C. Characterization of Cu-exchanged SSZ-13: A comparative FTIR, UV-Vis, and EPR study with Cu-ZSM-5 and Cu-β with similar Si/Al and Cu/Al ratios. Dalton Trans. 2013, 42, 12741–12761. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.D.; Peneau, V.; Ritterskamp, N.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. The Role of Copper Speciation in the Low Temperature Oxidative Upgrading of Short Chain Alkanes over Cu/ZSM-5 Catalysts. ChemPhysChem 2018, 19, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Dedecek, J.; Čapek, L.; Sazama, P.; Sobalik, Z.; Wichterlová, B. Control of metal ion species in zeolites by distribution of aluminium in the framework: From structural analysis to performance under real conditions of SCR-NOx and NO, N2O decomposition. Appl. Catal. A 2011, 391, 244–253. [Google Scholar] [CrossRef]
- Li, G.; Pidko, E.A.; van Santen, R.A.; Li, C.; Hensen, E.J.M. Stability of Extraframework Iron-Containing Complexes in ZSM-5 Zeolite. J. Phys. Chem. C 2013, 117, 413–426. [Google Scholar] [CrossRef]
- Hammond, C.; Dimitratos, N.; Jenkins, R.L.; Lopez-Sanchez, J.A.; Kondrat, S.A.; Hasbi ab Rahim, M.; Forde, M.M.; Thetford, A.; Taylor, S.H.; Hagen, H.; et al. Elucidation and Evolution of the Active Component within Cu/Fe/ZSM-5 for Catalytic Methane Oxidation: From Synthesis to Catalysis. ACS Catal. 2013, 3, 689–699. [Google Scholar] [CrossRef]
- Hammond, C.; Dimitratos, N.; Lopez-Sanchez, J.A.; Jenkins, R.L.; Whiting, G.; Kondrat, S.A.; ab Rahim, M.H.; Forde, M.M.; Thetford, A.; Hagen, H.; et al. Aqueous-Phase Methane Oxidation over Fe-MFI Zeolites; Promotion through Isomorphous Framework Substitution. ACS Catal. 2013, 3, 1835–1844. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Freakley, S.J.; Forde, M.M.; Peneau, V.; Jenkins, R.L.; Taylor, S.H.; Moulijn, J.A.; Morgan, D.J.; Hutchings, G.J. Low temperature catalytic partial oxidation of ethane to oxygenates by Fe– and Cu–ZSM-5 in a continuous flow reactor. J. Catal. 2015, 330, 84–92. [Google Scholar] [CrossRef]
- Kalamaras, C.; Palomas, D.; Bos, R.; Horton, A.; Crimmin, M.; Hellgardt, K. Selective Oxidation of Methane to Methanol Over Cu- and Fe-Exchanged Zeolites: The Effect of Si/Al Molar Ratio. Catal. Lett. 2016, 146, 483–492. [Google Scholar] [CrossRef]
- Peneau, V.; Armstrong, R.D.; Shaw, G.; Xu, J.; Jenkins, R.L.; Morgan, D.J.; Dimitratos, N.; Taylor, S.H.; Zanthoff, H.W.; Peitz, S.; et al. The Low-Temperature Oxidation of Propane by using H2O2 and Fe/ZSM-5 Catalysts: Insights into the Active Site and Enhancement of Catalytic Turnover Frequencies. ChemCatChem 2017, 9, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Wang, Y.; Nishitoba, T.; Kondo, J.N.; Yokoi, T. Selective oxidation of methane to methanol with H2O2 over an Fe-MFI zeolite catalyst using sulfolane solvent. Chem. Commun. 2019, 55, 2896–2899. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Boltenkov, V.V.; Babushkin, D.E.; Taran, O.P.; Parmon, V.N. Methane Oxidation by H2O2 over Different Cu-Species of Cu-ZSM-5 Catalysts. Top. Catal. 2020, 63, 203–221. [Google Scholar] [CrossRef]
- Xu, J.; Armstrong, R.D.; Shaw, G.; Dummer, N.F.; Freakley, S.J.; Taylor, S.H.; Hutchings, G.J. Continuous selective oxidation of methane to methanol over Cu- and Fe-modified ZSM-5 catalysts in a flow reactor. Catal. Today 2016, 270, 93–100. [Google Scholar] [CrossRef]







| Catalyst | Cu Content a (wt.%) | Fe Content a (wt.%) | BET Surface Area b (m2/g) | Pore Volume b (cm3/g) | Micropore Area c (m2/g) | Micropore Volume c (cm3/g) |
|---|---|---|---|---|---|---|
| H-ZSM-5 | - | 0.01 | 355 | 0.20 | 280 | 0.12 |
| 1.12%Cu/ZSM-5(SIE) | 1.12 | 0.01 | 304 | 0.21 | 198 | 0.09 |
| 0.54%Cu-0.56%Fe/ZSM-5(SIE) | 0.54 | 0.56 | 305 | 0.20 | 204 | 0.09 |
| 0.65%Fe/ZSM-5(SIE) | - | 0.65 | 368 | 0.23 | 268 | 0.12 |
| 1.13%Fe/ZSM-5(SIE) | - | 1.13 | 311 | 0.21 | 202 | 0.09 |
| 0.99%Cu/ZSM-5(WI) | 0.99 | 0.01 | 292 | 0.19 | 206 | 0.08 |
| 0.55%Cu-0.46%Fe/ZSM-5(WI) | 0.55 | 0.46 | 287 | 0.19 | 206 | 0.09 |
| 0.54%Fe/ZSM-5(WI) | - | 0.54 | 300 | 0.27 | 204 | 0.09 |
| 1.05%Fe/ZSM-5(WI) | - | 1.05 | 301 | 0.21 | 191 | 0.09 |
| 1.20%Cu/ZSM-5(IE) | 1.20 | 0.01 | 318 | 0.20 | 237 | 0.11 |
| 0.56%Cu-0.30%Fe/ZSM-5(IE) | 0.56 | 0.30 | 314 | 0.24 | 206 | 0.09 |
| 0.51%Fe/ZSM-5(IE) | - | 0.51 | 315 | 0.26 | 208 | 0.10 |
| 0.94%Fe/ZSM-5(IE) | - | 0.94 | 311 | 0.24 | 211 | 0.10 |
| Entry | Catalyst | The Fraction of Cu Leached |
|---|---|---|
| 1 | 1.12%Cu/ZSM-5(SIE) | 0.27 |
| 2 | 0.99%Cu/ZSM-5(WI) | 0.37 |
| 3 | 1.20%Cu/ZSM-5(IE) | 0.13 |
| 4 | 0.54%Cu-0.56%Fe/ZSM-5(SIE) | 0.47 |
| 5 | 0.55%Cu-0.46%Fe/ZSM-5(WI) | 0.55 |
| 6 | 0.56%Cu-0.30%Fe/ZSM-5(IE) | 0.55 |
| Entry | Temperature (°C) | Concentration of Each Metal in the Solution (ppm) | |
|---|---|---|---|
| Cu | Fe | ||
| 1 | 10 | 0.534 | 0.001 |
| 2 | 30 | 4.59 | 0.025 |
| 3 | 50 | 5.04 | 0.392 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Yang, G.S.; Park, E.D. Effects of Cu Species on Liquid-Phase Partial Oxidation of Methane with H2O2 over Cu-Fe/ZSM-5 Catalysts. Catalysts 2022, 12, 1224. https://doi.org/10.3390/catal12101224
Kim MS, Yang GS, Park ED. Effects of Cu Species on Liquid-Phase Partial Oxidation of Methane with H2O2 over Cu-Fe/ZSM-5 Catalysts. Catalysts. 2022; 12(10):1224. https://doi.org/10.3390/catal12101224
Chicago/Turabian StyleKim, Min Sik, Gun Sik Yang, and Eun Duck Park. 2022. "Effects of Cu Species on Liquid-Phase Partial Oxidation of Methane with H2O2 over Cu-Fe/ZSM-5 Catalysts" Catalysts 12, no. 10: 1224. https://doi.org/10.3390/catal12101224
APA StyleKim, M. S., Yang, G. S., & Park, E. D. (2022). Effects of Cu Species on Liquid-Phase Partial Oxidation of Methane with H2O2 over Cu-Fe/ZSM-5 Catalysts. Catalysts, 12(10), 1224. https://doi.org/10.3390/catal12101224