Single-Atom Transition Metal Photocatalysts for Hydrogen Evolution Reactions
Abstract
:1. Introduction
2. Fundamentals
3. Single-Atom Metal Photocatalyst Preparation
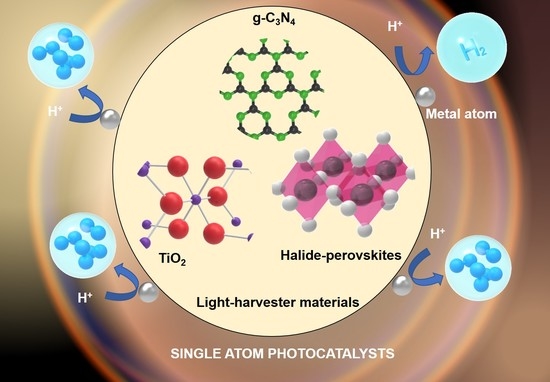
4. Single-Atom Photocatalysts
4.1. TiO2 Base
4.2. g-C3N4 Base
4.3. Other Host Materials
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef] [Green Version]
- Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165–9181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.P.; Tuan Nguyen, D.M.; Tran, D.L.; Le, H.K.; Vo, D.-V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Le, Q.V. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, X.; Sharman, E.; Yang, L.; Li, X.; Zhang, G.; Zhao, J.; Luo, Y.; Jiang, J. Isolating hydrogen from oxygen in photocatalytic water splitting with a carbon-quantum-dot/carbon-nitride hybrid. J. Mater. Chem. A 2019, 7, 6143–6148. [Google Scholar] [CrossRef]
- Lin, S.; Huang, H.; Ma, T.; Zhang, Y. Photocatalytic Oxygen Evolution from Water Splitting. Adv. Sci. 2021, 8, 2002458. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Santos, E.; Lundin, A.; Pötting, K.; Quaino, P.; Schmickler, W. Model for the electrocatalysis of hydrogen evolution. Phys. Rev. B 2009, 79, 235436. [Google Scholar] [CrossRef]
- Crawford, S.; Thimsen, E.; Biswas, P. Impact of Different Electrolytes on Photocatalytic Water Splitting. J. Electrochem. Soc. 2009, 156, H346. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Mahinpey, N.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Kinetic study of the effects of pH on the photocatalytic hydrogen production from alcohols. Int. J. Hydrogen Energy 2019, 44, 32030–32041. [Google Scholar] [CrossRef]
- Huang, B.; Rao, R.R.; You, S.; Hpone Myint, K.; Song, Y.; Wang, Y.; Ding, W.; Giordano, L.; Zhang, Y.; Wang, T.; et al. Cation- and pH-Dependent Hydrogen Evolution and Oxidation Reaction Kinetics. JACS Au 2021, 1, 1674–1687. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Minakata, D.; Chen, Y.; Crittenden, J.; Wang, P. The pH effects on H2 evolution kinetics for visible light water splitting over the Ru/(CuAg)0.15In0.3Zn1.4S2 photocatalyst. Int. J. Hydrogen Energy 2013, 38, 11727–11736. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Amorphous Catalysts and Electrochemical Water Splitting: An Untold Story of Harmony. Small 2020, 16, 1905779. [Google Scholar] [CrossRef]
- Jerkiewicz, G. Standard and Reversible Hydrogen Electrodes: Theory, Design, Operation, and Applications. ACS Catal. 2020, 10, 8409–8417. [Google Scholar] [CrossRef]
- Xia, Y.; Sayed, M.; Zhang, L.; Cheng, B.; Yu, J. Single-atom heterogeneous photocatalysts. Chem Catalysis 2021, 1, 1173–1214. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Z.; Yang, X.; Qian, X.; Liu, C.; Zhou, D.; Sun, T.; Zhang, M.; Wei, G.; Dissanayake, P.D.; et al. Recent advances in photocatalytic hydrogen evolution with high-performance catalysts without precious metals. Renew. Sustain. Energy Rev. 2020, 132, 110040. [Google Scholar] [CrossRef]
- Gellé, A.; Moores, A. Water splitting catalyzed by titanium dioxide decorated with plasmonic nanoparticles. Pure Appl. Chem. 2017, 89, 1817–1827. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, Y.; Ran, J.; Jaroniec, M.; Qiao, S.-Z. Single-Atom Photocatalysts for Emerging Reactions. ACS Central Science 2021, 7, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Hasija, V.; Patial, S.; Raizada, P.; Aslam Parwaz Khan, A.; Asiri, A.M.; Van Le, Q.; Nguyen, V.-H.; Singh, P. Covalent organic frameworks promoted single metal atom catalysis: Strategies and applications. Coord. Chem. Rev. 2022, 452, 214298. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Tu, X.; Liu, H.; Shu, M.; Si, R.; Ferguson, C.T.J.; Zhang, K.A.I.; Li, R. Single Atomically Anchored Cobalt on Carbon Quantum Dots as Efficient Photocatalysts for Visible Light-Promoted Oxidation Reactions. Chem. Mater. 2020, 32, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Baek, J.-B. Recent Advances in Noble Metal (Pt, Ru, and Ir)-Based Electrocatalysts for Efficient Hydrogen Evolution Reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-H.; Baar, M.; Blechert, S.; Antonietti, M. Facilitating room-temperature Suzuki coupling reaction with light: Mott-Schottky photocatalyst for C-C-coupling. Sci. Rep. 2013, 3, 1743. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Wang, Y.; Zhang, J.; Lin, Z.; Zheng, J.; Huang, F. Schottky or Ohmic Metal–Semiconductor Contact: Influence on Photocatalytic Efficiency of Ag/ZnO and Pt/ZnO Model Systems. ChemSusChem 2014, 7, 101–104. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, W.-H.; Long, R.; Prezhdo, O.V. Water Splitting with a Single-Atom Cu/TiO2 Photocatalyst: Atomistic Origin of High Efficiency and Proposed Enhancement by Spin Selection. JACS Au 2021, 1, 550–559. [Google Scholar] [CrossRef]
- Ge, Z.; Fu, B.; Zhao, J.; Li, X.; Ma, B.; Chen, Y. A review of the electrocatalysts on hydrogen evolution reaction with an emphasis on Fe, Co and Ni-based phosphides. J. Mater. Sci. 2020, 55, 14081–14104. [Google Scholar] [CrossRef]
- Wang, X.; Pan, H.; Sun, M.; Zhang, Y. Au single atom-anchored WO3/TiO2 nanotubes for the photocatalytic degradation of volatile organic compounds. J. Mater. Chem. A 2022, 10, 6078–6085. [Google Scholar] [CrossRef]
- Hou, T.; Peng, H.; Xin, Y.; Wang, S.; Zhu, W.; Chen, L.; Yao, Y.; Zhang, W.; Liang, S.; Wang, L. Fe Single-Atom Catalyst for Visible-Light-Driven Photofixation of Nitrogen Sensitized by Triphenylphosphine and Sodium Iodide. ACS Catal. 2020, 10, 5502–5510. [Google Scholar] [CrossRef]
- Xiong, X.; Mao, C.; Yang, Z.; Zhang, Q.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. Photocatalytic CO2 Reduction to CO over Ni Single Atoms Supported on Defect-Rich Zirconia. Adv. Energy Mater. 2020, 10, 2002928. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Popczyk, M.; Napłoszek, I.; Budniok, A. Intermetallic Compounds as Catalysts in the Reaction of Electroevolution/Absorption of Hydrogen. Solid State Phenom. 2015, 228, 16–22. [Google Scholar] [CrossRef]
- Xue, Z.-H.; Luan, D.; Zhang, H.; Lou, X.W. Single-atom catalysts for photocatalytic energy conversion. Joule 2022, 6, 92–133. [Google Scholar] [CrossRef]
- Gao, G.; Bottle, S.; Du, A. Understanding the activity and selectivity of single atom catalysts for hydrogen and oxygen evolution via ab initial study. Catal. Sci. Technol. 2018, 8, 996–1001. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the Electrochemistry of the Hydrogen-Evolution Reaction through Combining Experiment and Theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Z.-Y.; Wei, H.-T.; Deng, X.-Y.; Liu, Q.-J. DFT calculations for single-atom confinement effects of noble metals on monolayer g-C3N4 for photocatalytic applications. RSC Adv. 2021, 11, 4276–4285. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, D.; Zhou, Y.; Qin, F.; Wang, H.; Wang, W.; Yang, Y.; Zeng, G. Dual optimization approach to Mo single atom dispersed g-C3N4 photocatalyst: Morphology and defect evolution. Appl. Catal. B 2022, 303, 120904. [Google Scholar] [CrossRef]
- Song, W.; Lv, X.; Gao, Y.; Wang, L. Photocatalytic HER Performance of TiO2-supported Single Atom Catalyst Based on Electronic Regulation: A DFT Study. Chem. Res. Chin. Univ. 2022, 38, 1025–1031. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Santos, E.; Schmickler, W. d-Band Catalysis in Electrochemistry. ChemPhysChem 2006, 7, 2282–2285. [Google Scholar] [CrossRef]
- He, T.; Zhang, C.; Du, A. Single-atom supported on graphene grain boundary as an efficient electrocatalyst for hydrogen evolution reaction. Chem. Eng. Sci. 2019, 194, 58–63. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Meng, X.; Lin, H.; Hu, C.; Long, X.; Yang, S. Hydrogen evolution electrocatalysis with binary-nonmetal transition metal compounds. J. Mater. Chem. A 2017, 5, 5995–6012. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greeley, J.; Nørskov, J.K.; Kibler, L.A.; El-Aziz, A.M.; Kolb, D.M. Hydrogen Evolution Over Bimetallic Systems: Understanding the Trends. ChemPhysChem 2006, 7, 1032–1035. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, H.; Xiao, B.; Zhang, W.; Zhao, X.; Lv, T.; Thangamuthu, M.; Zhang, J.; Guo, Y.; et al. Single-atom Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56%. Nat. Commun. 2022, 13, 58. [Google Scholar] [CrossRef]
- Cha, G.; Hwang, I.; Hejazi, S.; Dobrota, A.S.; Pašti, I.A.; Osuagwu, B.; Kim, H.; Will, J.; Yokosawa, T.; Badura, Z.; et al. As a single atom Pd outperforms Pt as the most active co-catalyst for photocatalytic H2 evolution. iScience 2021, 24, 102938. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, G.; Guan, Z.; Zhao, Q.; Li, G.; Yang, J.; Li, Q.; Zou, Z. Anchoring Ni single atoms on sulfur-vacancy-enriched ZnIn2S4 nanosheets for boosting photocatalytic hydrogen evolution. J. Energy Chem. 2021, 58, 408–414. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, Z.; Han, C.B.; Ke, X.; Wang, C.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 2021, 12, 3783. [Google Scholar] [CrossRef]
- Vilé, G.; Sharma, P.; Nachtegaal, M.; Tollini, F.; Moscatelli, D.; Sroka-Bartnicka, A.; Tomanec, O.; Petr, M.; Filip, J.; Pieta, I.S.; et al. An Earth-Abundant Ni-Based Single-Atom Catalyst for Selective Photodegradation of Pollutants. Solar RRL 2021, 5, 2100176. [Google Scholar] [CrossRef]
- Zhang, P.; Zhan, X.; Xu, L.; Fu, X.; Zheng, T.; Yang, X.; Xu, Q.; Wang, D.; Qi, D.; Sun, T.; et al. Mass production of a single-atom cobalt photocatalyst for high-performance visible-light photocatalytic CO2 reduction. J. Mater. Chem. A 2021, 9, 26286–26297. [Google Scholar] [CrossRef]
- Zhou, X.; Hwang, I.; Tomanec, O.; Fehn, D.; Mazare, A.; Zboril, R.; Meyer, K.; Schmuki, P. Advanced Photocatalysts: Pinning Single Atom Co-Catalysts on Titania Nanotubes. Adv. Funct. Mater. 2021, 31, 2102843. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Z.; Yan, K.; Dong, H.; Dong, H.; Cui, J.; Gong, X.; Han, S.; Huang, L.; Wen, J. Single-atom-nickel photocatalytic site-selective sulfonation of enamides to access amidosulfones. Green Chem. 2021, 23, 2756–2762. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, L.; Xin, T.; Li, Y.; Wu, X.; Zhang, G. Single-atom V-N charge-transfer bridge on ultrathin carbon nitride for efficient photocatalytic H2 production and formaldehyde oxidation under visible light. Chem. Eng. J. 2022, 429, 132229. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom Catalysis Using Pt/Graphene Achieved through Atomic Layer Deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Song, Q.; Wu, J.; Lei, Q.; Li, J.; Zhang, W.; Huang, Z.; Kang, T.; Xu, H.; Wang, P.; et al. Anchoring Copper Single Atoms on Porous Boron Nitride Nanofiber to Boost Selective Reduction of Nitroaromatics. ACS Nano 2022, 16, 4152–4161. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Song, T.; Paik, U. TiO2 as an active or supplemental material for lithium batteries. J. Mater. Chem. A 2016, 4, 14–31. [Google Scholar] [CrossRef]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.-E.; Kang, K.-S.; Jung, H.; Yoon, J. Enhanced Photoelectrochemical Activity of TiO2 Nanotubes Decorated with Lanthanide Ions for Hydrogen Production. Catalysts 2022, 12, 866. [Google Scholar] [CrossRef]
- Son, Y.J.; Kang, J.S.; Yoon, J.; Kim, J.; Jeong, J.; Kang, J.; Lee, M.J.; Park, H.S.; Sung, Y.-E. Influence of TiO2 Particle Size on Dye-Sensitized Solar Cells Employing an Organic Sensitizer and a Cobalt(III/II) Redox Electrolyte. J. Phys. Chem. C 2018, 122, 7051–7060. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase – A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Yi, L.; Lan, F.; Li, J.; Zhao, C. Efficient Noble-Metal-Free Co-NG/TiO2 Photocatalyst for H2 Evolution: Synergistic Effect between Single-Atom Co and N-Doped Graphene for Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2018, 6, 12766–12775. [Google Scholar] [CrossRef]
- Cui, J.; Liang, S.; Zhang, J. A multifunctional material of two-dimensional g-C4N3/graphene bilayer. Phys. Chem. Chem. Phys. 2016, 18, 25388–25393. [Google Scholar] [CrossRef]
- Chen, J.; Fang, S.; Shen, Q.; Fan, J.; Li, Q.; Lv, K. Recent Advances of Doping and Surface Modifying Carbon Nitride with Characterization Techniques. Catalysts 2022, 12, 962. [Google Scholar] [CrossRef]
- Zuluaga, S.; Liu, L.H.; Shafiq, N.; Rupich, S.M.; Veyan, J.F.; Chabal, Y.J.; Thonhauser, T. Structural band-gap tuning in g-C3N4. Phys. Chem. Chem. Phys. 2015, 17, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Liu, E.; Shi, J.; Lin, X.; Sheng, L.; Zhang, M.; Wang, L.; Chen, J. A direct one-step synthesis of ultrathin g-C3N4 nanosheets from thiourea for boosting solar photocatalytic H2 evolution. Int. J. Hydrogen Energy 2019, 44, 7194–7204. [Google Scholar] [CrossRef]
- Antil, B.; Kumar, L.; Ranjan, R.; Shenoy, S.; Tarafder, K.; Gopinath, C.S.; Deka, S. One-Dimensional Multichannel g-C3N4.7 Nanostructure Realizing an Efficient Photocatalytic Hydrogen Evolution Reaction and Its Theoretical Investigations. ACS Appl. Energy Mater. 2021, 4, 3118–3129. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging Chemical Functionalization of g-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Thomas, A.; Fu, X.; Antonietti, M. Metal-Containing Carbon Nitride Compounds: A New Functional Organic–Metal Hybrid Material. Adv. Mater. 2009, 21, 1609–1612. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wang, D.; Lin, Y.; Liu, W.; Cao, L.; Liu, X.; Zhang, W.; Mou, X.; Fang, S.; Shen, X.; et al. Single Pt Atom with Highly Vacant d-Orbital for Accelerating Photocatalytic H2 Evolution. ACS Appl. Energy Mater. 2018, 1, 6082–6088. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Guo, Z.; Song, Y.; Tang, S.; Ma, Y.; Xing, X.; Wang, Q. Synthesis of Atomically Thin g-C3N4 Nanosheets via Supercritical CO2 Doping with Single-Atom Cobalt for Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 52560–52570. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis — Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Li, W.; Lin, Z.; Yang, G. A 2D self-assembled MoS2/ZnIn2S4 heterostructure for efficient photocatalytic hydrogen evolution. Nanoscale 2017, 9, 18290–18298. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-Mediated Electron-Hole Separation in One-Unit-Cell ZnIn2S4 Layers for Boosted Solar-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Xie, J.; Zhang, X.; Liu, Q.; Yao, T.; Wei, S.; Zhang, Q.; Xie, Y. Enhanced Photoexcited Carrier Separation in Oxygen-Doped ZnIn2S4 Nanosheets for Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 6716–6720. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Wang, X.; Hu, J.; Zhang, J.; Zheng, L.; Mao, L.; Zheng, H.; Zhu, M. Protruding Pt single-sites on hexagonal ZnIn2S4 to accelerate photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 1287. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Q.; Zhang, Q.; Lou, Z.; Liu, K.; Ma, Y.; Wang, Z.; Zheng, Z.; Cheng, H.; Liu, Y.; et al. An organometal halide perovskite supported Pt single-atom photocatalyst for H2 evolution. Energy Environ. Sci. 2022, 15, 1271–1281. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Zhang, A.; Cheng, T.; Xi, X.; Zhang, J. Platinum Single Atoms Anchored on a Covalent Organic Framework: Boosting Active Sites for Photocatalytic Hydrogen Evolution. ACS Catal. 2021, 11, 13266–13279. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, P.; Wu, Y.; Zhang, Q.; Wu, Q.; Wang, Z.; Zheng, Z.; Liu, Y.; Dai, Y.; Huang, B. Lead-Free Halide Perovskite Cs3Bi2xSb2–2xI9 (x ≈ 0.3) Possessing the Photocatalytic Activity for Hydrogen Evolution Comparable to that of (CH3NH3)PbI3. Adv. Mater. 2020, 32, 2001344. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Zhang, H.; Yu, W.; Wang, X.; Zhao, Y.; Zong, X.; Li, C. Dynamic Interaction between Methylammonium Lead Iodide and TiO2 Nanocrystals Leads to Enhanced Photocatalytic H2 Evolution from HI Splitting. ACS Energy Lett. 2018, 3, 1159–1164. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Chen, R.; Zhang, H.; Wang, X.; Wang, J.; Zhang, J.; Mu, L.; Wu, K.; Fan, F.; et al. Promoting Photocatalytic H2 Evolution on Organic–Inorganic Hybrid Perovskite Nanocrystals by Simultaneous Dual-Charge Transportation Modulation. ACS Energy Lett. 2019, 4, 40–47. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, G.; Li, Z.; Lou, Y.; Chen, J.; Zhao, Y. Stable Lead-Free (CH3NH3)3Bi2I9 Perovskite for Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2019, 7, 15080–15085. [Google Scholar] [CrossRef]
- Romani, L.; Malavasi, L. Solar-Driven Hydrogen Generation by Metal Halide Perovskites: Materials, Approaches, and Mechanistic View. ACS Omega 2020, 5, 25511–25519. [Google Scholar] [CrossRef]
- Ricciarelli, D.; Kaiser, W.; Mosconi, E.; Wiktor, J.; Ashraf, M.W.; Malavasi, L.; Ambrosio, F.; De Angelis, F. Reaction Mechanism of Photocatalytic Hydrogen Production at Water/Tin Halide Perovskite Interfaces. ACS Energy Lett. 2022, 7, 1308–1315. [Google Scholar] [CrossRef]
- Yu, J.; Xu, X. Expediting H2 Evolution over MAPbI3 with a Nonnoble Metal Cocatalyst Mo2C under Visible Light. Energy Mater. Adv. 2022, 2022, 9836095. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, H.; Chao, Y.; Zhang, Q.; Zhang, W.; Lv, F.; Gu, L.; Zhao, Q.; Wang, N.; Wang, J.; et al. Single-atom Pt-I3 sites on all-inorganic Cs2SnI6 perovskite for efficient photocatalytic hydrogen production. Nat. Commun. 2021, 12, 4412. [Google Scholar] [CrossRef]
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Cui, K.; Zhong, W.; Li, L.; Zhuang, Z.; Li, L.; Bi, J.; Yu, Y. Well-Defined Metal Nanoparticles@Covalent Organic Framework Yolk–Shell Nanocages by ZIF-8 Template as Catalytic Nanoreactors. Small 2019, 15, 1804419. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Y.; Wan, S.; McCaffrey, R.; Jin, Y.; Gu, H.; Zhang, W. Synthesis of Ultrafine and Highly Dispersed Metal Nanoparticles Confined in a Thioether-Containing Covalent Organic Framework and Their Catalytic Applications. J. Am. Chem. Soc. 2017, 139, 17082–17088. [Google Scholar] [CrossRef]
- Zhong, W.; Sa, R.; Li, L.; He, Y.; Li, L.; Bi, J.; Zhuang, Z.; Yu, Y.; Zou, Z. A Covalent Organic Framework Bearing Single Ni Sites as a Synergistic Photocatalyst for Selective Photoreduction of CO2 to CO. J. Am. Chem. Soc. 2019, 141, 7615–7621. [Google Scholar] [CrossRef]
- Pachfule, P.; Acharjya, A.; Roeser, J.; Langenhahn, T.; Schwarze, M.; Schomacker, R.; Thomas, A.; Schmidt, J. Diacetylene Functionalized Covalent Organic Framework (COF) for Photocatalytic Hydrogen Generation. J. Am. Chem. Soc. 2018, 140, 1423–1427. [Google Scholar] [CrossRef]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B.V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef] [Green Version]
- Chai, S.; Chen, X.; Zhang, X.; Fang, Y.; Sprick, R.S.; Chen, X. Rational design of covalent organic frameworks for efficient photocatalytic hydrogen peroxide production. Environ. Sci. Nano 2022, 9, 2464–2469. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Li, H.; Wu, K.; Wang, J.; Yang, Q. Covalent organic frameworks with high quantum efficiency in sacrificial photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 2357. [Google Scholar] [CrossRef]
- Li, X.; Kawai, K.; Fujitsuka, M.; Osakada, Y. COF-based photocatalyst for energy and environment applications. Surf. Interfaces 2021, 25, 101249. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.-J.; Zong, M.-Y.; Xu, J.; Wang, D.-H.; Bu, X.-H. Energy Level Engineering: Ru Single Atom Anchored on Mo-MOF with a [Mo8O26(im)2]4– Structure Acts as a Biomimetic Photocatalyst. ACS Catal. 2022, 12, 7960–7974. [Google Scholar] [CrossRef]
- Ma, X.; Yong, X.; Jian, C.-c.; Zhang, J. Transition Metal-Functionalized Janus MoSSe Monolayer: A Magnetic and Efficient Single-Atom Photocatalyst for Water-Splitting Applications. J. Phys. Chem. C 2019, 123, 18347–18354. [Google Scholar] [CrossRef]
- Han, A.; Zhou, X.; Wang, X.; Liu, S.; Xiong, Q.; Zhang, Q.; Gu, L.; Zhuang, Z.; Zhang, W.; Li, F.; et al. One-step synthesis of single-site vanadium substitution in 1T-WS2 monolayers for enhanced hydrogen evolution catalysis. Nat. Commun. 2021, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Si, H.; Zhang, Q.; Wu, J.; Gao, L.; Wei, X.; Sun, Y.; Liao, Q.; Zhang, Z.; et al. Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2. J. Am. Chem. Soc. 2020, 142, 4298–4308. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Liu, B.; Luo, M.; Ning, S.; Peng, M.; Zhao, Y.; Lu, Y.-R.; Chan, T.-S.; de Groot, F.M.F.; Tan, Y. Single platinum atoms embedded in nanoporous cobalt selenide as electrocatalyst for accelerating hydrogen evolution reaction. Nat. Commun. 2019, 10, 1743. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhang, S.; Pan, H.; Xiao, S.; Guo, Z.; Tang, L.; Khan, U.; Ding, B.-F.; Li, M.; Cai, Z.; et al. Unsaturated Single Atoms on Monolayer Transition Metal Dichalcogenides for Ultrafast Hydrogen Evolution. ACS Nano 2020, 14, 767–776. [Google Scholar] [CrossRef]
- Lv, S.; Pei, M.; Liu, Y.; Si, Z.; Wu, X.; Ran, R.; Weng, D.; Kang, F. An isolation strategy to anchor atomic Ni or Co cocatalysts on TiO2(A) for photocatalytic hydrogen production. Nano Res. 2022, 15, 5848–5856. [Google Scholar] [CrossRef]
- Liu, G.; Lv, H.; Zeng, Y.; Yuan, M.; Meng, Q.; Wang, Y.; Wang, C. Single-Atom Pd–N3 Sites on Carbon-Deficient g-C3N4 for Photocatalytic H2 Evolution. Trans. Tianjin Univ. 2021, 27, 139–146. [Google Scholar] [CrossRef]
- Guan, Z.; Pan, J.; Li, Q.; Li, G.; Yang, J. Boosting Visible-Light Photocatalytic Hydrogen Evolution with an Efficient CuInS2/ZnIn2S4 2D/2D Heterojunction. ACS Sustain. Chem. Eng. 2019, 7, 7736–7742. [Google Scholar] [CrossRef]
- Ding, C.; Shi, J.; Wang, Z.; Li, C. Photoelectrocatalytic Water Splitting: Significance of Cocatalysts, Electrolyte, and Interfaces. ACS Catal. 2017, 7, 675–688. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Shi, W.; Shao, C.; Wang, S.; Ding, C.; Liu, T.; Fan, F.; Shi, J.; Li, C. Crystallographic-Orientation-Dependent Charge Separation of BiVO4 for Solar Water Oxidation. ACS Energy Lett. 2019, 4, 825–831. [Google Scholar] [CrossRef]
- Sun, H.; Hua, W.; Li, Y.; Wang, J.-G. Conformal coating of superhydrophilic metal-organic complex toward substantially improved photoelectrochemical water oxidation. Chem. Eng. J. 2022, 427, 131004. [Google Scholar] [CrossRef]
- Sun, H.; Hua, W.; Liang, S.; Li, Y.; Wang, J.-G. A self-adaptive semiconductor–liquid junction for highly active and stable solar water splitting. J. Mater. Chem. A 2022, 10, 20414–20423. [Google Scholar] [CrossRef]
- Lee, W.H.; Ko, Y.-J.; Kim, J.-Y.; Min, B.K.; Hwang, Y.J.; Oh, H.-S. Single-atom catalysts for the oxygen evolution reaction: Recent developments and future perspectives. Chem. Commun. 2020, 56, 12687–12697. [Google Scholar] [CrossRef]
- Yin, J.; Jin, J.; Lu, M.; Huang, B.; Zhang, H.; Peng, Y.; Xi, P.; Yan, C.H. Iridium Single Atoms Coupling with Oxygen Vacancies Boosts Oxygen Evolution Reaction in Acid Media. J. Am. Chem. Soc. 2020, 142, 18378–18386. [Google Scholar] [CrossRef]







| Single-Atom Metal | Host Materials | Synthesis Method | Ref |
|---|---|---|---|
| Cu, Co, Ni, Fe, Mn, Zn, Pt | TiO2 | Absorption on MOF and calcination | [47] |
| Ni | TiO2 | Refining by hydrothermal | [54] |
| Ni | ZnIn2S4 | Deposition: electrostatic deposition + hydrothermal | [49] |
| Pd, Pt, Au | TiO2 | Absorption: Immersing in salts | [48] |
| Ni | C3N4 | Refining by sodium borohydride | [51] |
| Co | N-Carbon | Refining by calcination | [52] |
| Pt | Ni/NiO on Ag NWs | Electrodeposition | [50] |
| V | C3N4 | Refining by calcination | [55] |
| Ir | TiO2 | Deposition in dark | [53] |
| Pt | Graphene | Deposition: Atomic layer deposition | [56] |
| Cu | BN | Refining by calcination | [57] |
| Metal Atom | Host Materials | Photocurrent (μA cm–2) | H2 Evolution Rate (mmol g−1·h−1) | Reference |
|---|---|---|---|---|
| Co | TiO2 nanobelt | ~83 | ~0.677 | [65] |
| - | ~28 | ~0.0217 | ||
| Co | TiO2 nanosheets (NSs) | n/a | ~2.9 | [107] |
| Ni | n/a | ~1.0 | ||
| - | n/a | ~0.04 | ||
| Cu | TiO2 (derived from MIL125-Ti) | ~3.0 | ~101.7 | [47] |
| Pt | n/a | ~95.0 | ||
| - | ~1.0 | ~4.2 | ||
| Pt | g-C3N4 NSs | n/a | ~0.042 | [74] |
| - | n/a | ~0.001 | ||
| Co | P-doped C3N4 ultrathin NSs | 6.0 | ~3.7 | [75] |
| - | 4.0 | ~0.4 | ||
| Pd | Carbon deficient g-C3N4 | n/a | ~2.8 | [108] |
| - | n/a | ~0.115 | ||
| V | Polymeric C3N4 | 4.6 | ~5.0 | [55] |
| - | 1.7 | ~1.5 | ||
| Ni | Sulfur-vacancy-enrich ZnI2S4 | ~22.5 | ~0.089 | [49] |
| - | ~10.0 | ~0.04 | ||
| Pt | ZnI2S4 NSs | ~17.0 | 0.35 | [80] |
| - | ~4.0 | 0.02 | ||
| - | CuInS2/ZnIn2S4 | ~0.75 | ~0.34 | [109] |
| Pt | FAPbBr3−xIx | ~12.0 | ~0.7 | [81] |
| - | ~3.0 | ~0.05 | ||
| - | Cs3Bi0.6Sb1.4I9 | ~20 | n/a | [84] |
| Pt | TpPa COF | n/a | ~0.72 | [82] |
| - | n/a | 0.015 | ||
| - | TP-BDDA COF | n/a | 0.32 | [96] |
| - | TP-DTP COF | n/a | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.P.; Kim, I.T. Single-Atom Transition Metal Photocatalysts for Hydrogen Evolution Reactions. Catalysts 2022, 12, 1304. https://doi.org/10.3390/catal12111304
Nguyen TP, Kim IT. Single-Atom Transition Metal Photocatalysts for Hydrogen Evolution Reactions. Catalysts. 2022; 12(11):1304. https://doi.org/10.3390/catal12111304
Chicago/Turabian StyleNguyen, Thang Phan, and Il Tae Kim. 2022. "Single-Atom Transition Metal Photocatalysts for Hydrogen Evolution Reactions" Catalysts 12, no. 11: 1304. https://doi.org/10.3390/catal12111304
APA StyleNguyen, T. P., & Kim, I. T. (2022). Single-Atom Transition Metal Photocatalysts for Hydrogen Evolution Reactions. Catalysts, 12(11), 1304. https://doi.org/10.3390/catal12111304