Visible-Light-Sensitive Polymerizable and Polymeric Triazine-Based Photoinitiators with Enhanced Migration Stability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Triazine Derivatives
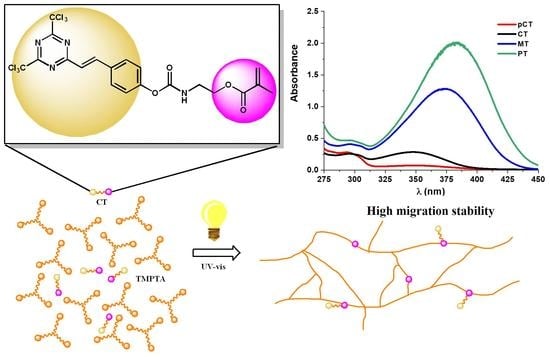
2.2. Light Absorption Properties of MT, PT, CT, and pCT
2.3. Photoinitiation Abilities of Triazine Derivatives
2.4. Migration of Photoinitiators from Photocured Samples
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. 4-(2-(4,6-Bis(trichloromethyl)-1,3,5-triazin-2-yl)vinyl)phenol (PT)
3.2.2. Polymerizable 2-(((4-(2-(4,6-Bis(trichloromethyl)-1,3,5-triazin-2-yl)vinyl)phenoxy)carbonyl)amino)ethyl Methacrylate (CT)
3.2.3. Polymerized 2-(((4-(2-(4,6-Bis(trichloromethyl)-1,3,5-triazin-2-yl)vinyl)phenoxy)carbonyl)amino)ethyl Methacrylate (pCT)
3.3. Irradiation Sources
3.4. Characterizations
3.4.1. Fourier-Transform Infrared Spectroscopy
3.4.2. Ultraviolet-Visible (UV-vis) Measurements
3.4.3. Gel Permeation Chromatography Analysis
3.4.4. NMR Spectroscopy
3.5. Photopolymerization Experiments
3.6. Migration Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Cataldi, A.; Corcione, C.E.; Frigione, M.; Pegoretti, A. Photocurable resin/nanocellulose composite coatings for wood protection. Prog. Org. Coat. 2017, 106, 128–136. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Leibfarth, F.; Mattson, K.; Fors, B.P.; Collins, H.A.; Hawker, C.J. External Regulation of Controlled Polymerizations. Angew. Chem. Int. Ed. 2013, 52, 199–210. [Google Scholar] [CrossRef]
- Liu, C.; Li, T.; Zhang, J.; Chen, S.; Xu, Z.; Zhang, A.; Zhang, D. Preparation and properties of phosphorous–nitrogen containing UV-curable polymeric coatings based on thiol–ene click reaction. Prog. Org. Coat. 2016, 90, 21–27. [Google Scholar] [CrossRef]
- Chiulan, I.; Heggset, E.B.; Voicu, Ş.I.; Chinga-Carrasco, G. Photopolymerization of Bio-Based Polymers in a Biomedical Engi-neering Perspective. Biomacromolecules 2021, 22, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, Y.; Cai, S.; Yang, J. Three-Dimensional Printing of Liquid Crystal Elastomers and Their Applications. ACS Appl. Polym. Mater. 2022, 4, 3153–3168. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Anastasaki, A.; Nikolaou, V.; Zhang, Q.; Burns, J.; Samanta, S.R.; Waldron, C.; Haddleton, A.J.; McHale, R.; Fox, D.; Percec, V.; et al. Copper(II)/Tertiary Amine Synergy in Photoinduced Living Radical Polymerization: Accelerated Synthesis of ω-Functional and α,ω-Heterofunctional Poly(acrylates). J. Am. Chem. Soc. 2014, 136, 1141–1149. [Google Scholar] [CrossRef]
- Lee, E.K.; Park, C.H.; Lee, J.; Lee, H.R.; Yang, C.; Oh, J.H. Chemically robust ambipolar organic transistor array directly patterned by photolithography. Adv. Mater. 2017, 29, 1605282. [Google Scholar] [CrossRef] [PubMed]
- Kütahya, C.; Wang, P.; Li, S.; Liu, S.; Li, J.; Chen, Z.; Strehmel, B. Carbon Dots as a Promising Green Photocatalyst for Free Radical and ATRP-Based Radical Photopolymerization with Blue LEDs. Angew. Chem. Int. Ed. 2020, 59, 3166–3171. [Google Scholar] [CrossRef]
- Shanmugam, S.; Boyer, C. Organic photocatalysts for cleaner polymer synthesis. Science 2016, 352, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Theriot, J.C.; Lim, C.-H.; Yang, H.; Ryan, M.D.; Musgrave, C.B.; Miyake, G.M. Organocatalyzed atom transfer radical polymer-ization driven by visible light. Science 2016, 352, 1082–1086. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Liao, Q.; Xu, C.; Wu, X.; Xiong, Y.; Li, Z.; Tang, H. Structural Effects of Cinnamoyl-Indanone-Based Photobleachable Free Radical Visible Initiators. ACS Appl. Polym. Mater. 2022, 4, 6466–6476. [Google Scholar] [CrossRef]
- Chi, T.; Somers, P.; Wilcox, D.A.; Schuman, A.J.; Johnson, J.E.; Liang, Z.; Pan, L.; Xu, X.; Boudouris, B.W. Substituted Thioxan-thone-Based Photoinitiators for Efficient Two-Photon Direct Laser Writing Polymerization with Two-Color Resolution. ACS Appl. Polym. Mater. 2021, 3, 1426–1435. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Shi, M.; Zhang, Y.; Zhao, Y. Study on a polymerizable visible light initiator for fabrication of biosafety materials. Polym. Chem. 2019, 10, 2273–2281. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Xiong, Y.; Yang, J.; Tang, H. Thioxanthone based one-component polymerizable visible light photoinitiator for free radical polymerization. RSC Adv. 2016, 6, 66098–66107. [Google Scholar] [CrossRef]
- Liska, R. Industrial Photoinitiators: A Technical Guide by W. Arthur Green. ChemPhysChem 2011, 12, 1389. [Google Scholar] [CrossRef]
- Ay, E.; Raad, Z.; Dautel, O.; Dumur, F.; Wantz, G.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Oligomeric Photocatalysts in Photoredox Catalysis: Toward High Performance and Low Migration Polymerization Photoinitiating Systems. Macromolecules 2016, 49, 2124–2134. [Google Scholar] [CrossRef]
- Johns, S.; Jickells, S.; Read, W.; Castle, L. Studies on functional barriers to migration. Components from cartonboard to food during frozen storage and microwave heating. Packag. Technol. Sci. 2000, 13, 99–104. [Google Scholar] [CrossRef]
- Papilloud, S.; Baudraz, D. Analysis of food packaging UV inks for chemicals with potential to migrate into food simulants. Food Addit. Contam. 2002, 19, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Pastorelli, S.; Cruz, J.; Simoneau, C.; Castanheira, I.; Paseiro-Losada, P. Development of an Analytical Method for the Determination of Photoinitiators Used for Food Packaging Materials with Potential to Migrate into Milk. J. Dairy Sci. 2008, 91, 900–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastorelli, S.; Sanches-Silva, A.; Cruz, J.M.; Simoneau, C.; Losada, P.P. Study of the migration of benzophenone from printed paperboard packages to cakes through different plastic films. Eur. Food Res. Technol. 2008, 227, 1585–1590. [Google Scholar] [CrossRef]
- Rodriguez-Bernaldo De Quirós, A.R.-B.; Paseiro-Cerrato, R.; Pastorelli, S.; Koivikko, R.; Simoneau, C.; Paseiro-Losada, P. Migration of Photoinitiators by Gas Phase into Dry Foods. J. Agric. Food Chem. 2009, 57, 10211–10215. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Andre, C.; Castanheira, I.; Cruz, J.M.; Pastorelli, S.; Simoneau, C.; Paseiro-Losada, P. Study of the Migration of Photoinitiators Used in Printed Food-Packaging Materials into Food Simulants. J. Agric. Food Chem. 2009, 57, 9516–9523. [Google Scholar] [CrossRef]
- Shen, D.-X.; Lian, H.-Z.; Ding, T.; Xu, J.-Z.; Shen, C.-Y. Determination of low-level ink photoinitiator residues in packaged milk by solid-phase extraction and LC-ESI/MS/MS using triple-quadrupole mass analyzer. Anal. Bioanal. Chem. 2009, 395, 2359–2370. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, X.; Huang, X.; Zhang, Y.; Shi, M.; Zhao, Y. High-efficient lignin-based polymerizable macromolecular photoinitiator with UV-blocking property for visible light polymerization. Int. J. Biol. Macromol. 2022, 204, 234–244. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Frigoli, M.; Tehfe, M.-A.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Naphthalimide based methacrylated photoinitiators in radical and cationic photopolymerization under visible light. Polym. Chem. 2013, 4, 5440–5448. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B. A Highly Efficient Polymerizable Photoinitiator Comprising Benzophenone, Thio Moieties, and N-Arylmaleimide. Macromol. Chem. Phys. 2011, 212, 88–95. [Google Scholar] [CrossRef]
- Liang, S.; Yang, Y.; Zhou, H.; Li, Y.; Wang, J. Novel polymerizable HMPP-type photoinitiator with carbamate: Synthesis and photoinitiating behaviors. Prog. Org. Coat. 2017, 110, 128–133. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Atif, M.; Bongiovanni, R.; Li, G.; Xue, Z.; Yang, X. One-component photoinitiator based on benzophenone and sesamol. Polym. Adv. Technol. 2018, 29, 2264–2272. [Google Scholar] [CrossRef]
- Kreutzer, J.; Yagci, Y. One-component, double-chromophoric thioxanthone photoinitiators for free radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3475–3482. [Google Scholar] [CrossRef]
- Hutchison, J.B.; Stark, P.F.; Hawker, C.J.; Anseth, K.S. Polymerizable Living Free Radical Initiators as a Platform To Synthesize Functional Networks. Chem. Mater. 2005, 17, 4789–4797. [Google Scholar] [CrossRef]
- Wang, J.; Stanic, S.; Altun, A.A.; Schwentenwein, M.; Dietliker, K.; Jin, L.; Stampfl, J.; Baudis, S.; Liska, R.; Grützmacher, H. A highly efficient waterborne photoinitiator for visible-light-induced three-dimensional printing of hydrogels. Chem. Commun. 2018, 54, 920–923. [Google Scholar] [CrossRef]
- Sandmeier, M.; Paunović, N.; Conti, R.; Hofmann, L.; Wang, J.; Luo, Z.; Masania, K.; Wu, N.; Kleger, N.; Coulter, F.B.; et al. Solvent-Free Three-Dimensional Printing of Biodegradable Elastomers Using Liquid Macrophotoinitiators. Macromolecules 2021, 54, 7830–7839. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Synthesis and photopolymerization of novel UV-curable macro-photoinitiators. Prog. Org. Coat. 2020, 141, 105546. [Google Scholar] [CrossRef]
- Yang, J.; Liao, W.; Xiong, Y.; Wang, X.; Li, Z.; Tang, H. A multifunctionalized macromolecular silicone-naphthalimide visible photoinitiator for free radical polymerization. Prog. Org. Coat. 2018, 115, 151–158. [Google Scholar] [CrossRef]
- Gacal, B.; Akat, H.; Balta, D.K.; Arsu, N.; Yagci, Y. Synthesis and Characterization of Polymeric Thioxanthone Photoinitatiors via Double Click Reactions. Macromolecules 2008, 41, 2401–2405. [Google Scholar] [CrossRef]
- Wang, C.; Venditti, R.A. UV Cross-Linkable Lignin Thermoplastic Graft Copolymers. ACS Sustain. Chem. Eng. 2015, 3, 1839–1845. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the development of polymeric and multifunctional photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, J.; Stachurski, Z.H.; Holl, M.M.B.; Xiao, P. Visible-Light-Sensitive Triazine-Coated Silica Nanoparticles: A Dual Role Approach to Polymer Nanocomposite Materials with Enhanced Properties. ACS Appl. Mater. Interfaces 2021, 13, 46033–46042. [Google Scholar] [CrossRef]
- Fan, Y.; Song, Y.; He, N.; Cheng, F.; Jiao, X.; Lai, G.; Hua, X.; Yang, X. High Efficiency and Low Migration Hyperbranched Silicone Contain Macrophotoinitiators for UV-Cured Transparent Coatings. Polymers 2020, 12, 3005. [Google Scholar] [CrossRef] [PubMed]
- Nugent, L.J.; Jain, R.K. Extravascular diffusion in normal and neoplastic tissues. Cancer Res. 1984, 44, 238–244. [Google Scholar] [PubMed]
- Radomsky, M.L.; Whaley, K.J.; A Cone, R.; Saltzman, W.M. Macromolecules released from polymers: Diffusion into unstirred fluids. Biomaterials 1990, 11, 619–624. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. A known photoinitiator for a novel technology: 2-(4-methoxystyryl)-4,6-bis(trichloromethyl)-1,3,5-triazine for near UV or visible LED. Polym. Chem. 2014, 5, 6019–6026. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity, and Efficiency, 1st ed; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Fouassier, J.-P. Photoinitiation, Photopolymerization, and Photocuring: Fundamentals and Applications; Carl Hanser Verlag GmbH & Co.: Munich, Germany, 1995. [Google Scholar]
- Crivello, J. Photoinitiators for Free Radical Cationic and Anionic Photopolymerization; John Wiley & Sons: Chichester, UK, 1998. [Google Scholar]
- Dietliker, K. A Compilation of Photoinitiators Commercially Available for UV Today; SITA: London, UK, 2002. [Google Scholar]
- Ullrich, G.; Ganster, B.; Salz, U.; Moszner, N.; Liska, R. Photoinitiators with functional groups. IX. Hydrophilic bisacylphosphine oxides for acidic aqueous formulations. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1686–1700. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly efficient wa-ter-soluble visible light photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Lai, H.; Zhu, D.; Xiao, P. Yellow triazine as an efficient photoinitiator for polymerization and 3D printing under LEDs. Macromol. Chem. Phys. 2019, 220, 1900315. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lai, H.; Zeng, B.; Wang, L.; Xing, F.; Xiao, P. Photoinduced free radical-releasing systems and their anticancer properties. Photochem. Photobiol. Sci. 2022, 21, 1405–1417. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach, Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Antosiewicz, J.M.; Shugar, D. UV–Vis spectroscopy of tyrosine side-groups in studies of protein structure. Part 1: Basic principles and properties of tyrosine chromophore. Biophys. Rev. 2016, 8, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.D. Effects of Substituents on Ultra-violet Absorption Spectra. Nature 1952, 169, 286–287. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Li, M.; Wang, E. Host/guest complex of Me-β-CD/2,2-dimethoxy-2-phenyl acetophenone for initiation of aqueous photopolymerization: Kinetics and mechanism. Polymer 2005, 46, 11934–11939. [Google Scholar] [CrossRef]
- Lai, H.; Zhu, D.; Peng, X.; Zhang, J.; Lalevée, J.; Xiao, P. N-Aryl glycines as versatile initiators for various polymerizations. Polym. Chem. 2021, 12, 1991–2000. [Google Scholar] [CrossRef]








| Photoinitiators | λmax (nm) | εmax (M−1 cm−1) | ε400 nm (M−1 cm−1) | ε410 nm (M−1 cm−1) |
|---|---|---|---|---|
| MT | 380 | 29,400 | 21,500 | 14,000 |
| PT | 385 | 26,000 | 23,900 | 19,000 |
| CT | 350 | 29,900 | 3300 | 1500 |
| pCT | 350 | 24,900 | 5100 | 3100 |
| Photoinitiators a | LED at 400 nm | LED at 410 nm | ||
|---|---|---|---|---|
| Cb | (Rp/[C=C]) × 100 c | Cb | (Rp/[C=C]) × 100 c | |
| MT | 35.6% | 4.21 s−1 | 45.0% | 10.91 s−1 |
| PT | 36.2% | 3.31 s−1 | 46.4% | 7.34 s−1 |
| CT | 15.3% | 0.83 s−1 | 40.5% | 5.59 s−1 |
| pCT | 24.9% | 0.18 s−1 | 22.5% | 2.97 s−1 |
| Photoinitiators | LED at 400 nm | LED at 410 nm |
|---|---|---|
| MT | 41.03% | 3.75% |
| PT | 72.88% | 11.91% |
| CT | 13.35% | 1.55% |
| pCT | 2.88% | 0.61% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhu, D.; Peng, X.; Xiao, P. Visible-Light-Sensitive Polymerizable and Polymeric Triazine-Based Photoinitiators with Enhanced Migration Stability. Catalysts 2022, 12, 1305. https://doi.org/10.3390/catal12111305
Li L, Zhu D, Peng X, Xiao P. Visible-Light-Sensitive Polymerizable and Polymeric Triazine-Based Photoinitiators with Enhanced Migration Stability. Catalysts. 2022; 12(11):1305. https://doi.org/10.3390/catal12111305
Chicago/Turabian StyleLi, Liqiang, Di Zhu, Xiaotong Peng, and Pu Xiao. 2022. "Visible-Light-Sensitive Polymerizable and Polymeric Triazine-Based Photoinitiators with Enhanced Migration Stability" Catalysts 12, no. 11: 1305. https://doi.org/10.3390/catal12111305
APA StyleLi, L., Zhu, D., Peng, X., & Xiao, P. (2022). Visible-Light-Sensitive Polymerizable and Polymeric Triazine-Based Photoinitiators with Enhanced Migration Stability. Catalysts, 12(11), 1305. https://doi.org/10.3390/catal12111305