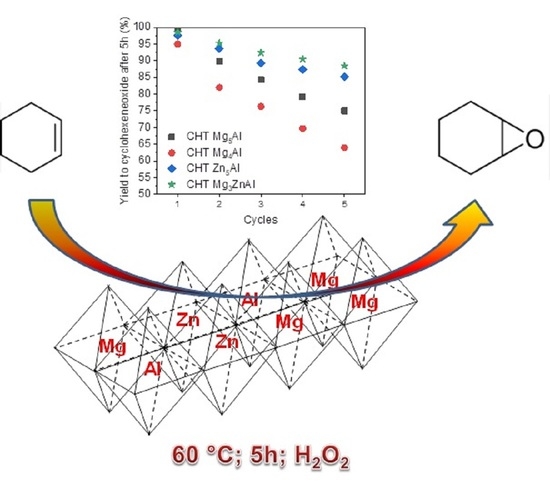
Green Epoxidation of Olefins with ZnxAl/MgxAl-LDH Compounds: Influence of the Chemical Composition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.2. Catalytic Activity
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
3.4. Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Romero, M.D.; Calles, J.A.; Ocaña, M.A.; Gómez, J.M. Epoxidation of cyclohexene over basic mixed oxides derived from hydrotalcite materials: Activating agent, solvent and catalyst reutilization. Microporous Mesoporous Mater. 2008, 111, 243–253. [Google Scholar] [CrossRef]
- Schmidt, F. New catalyst preparation technologies- observed from an industrial viewpoint. Appl. Catal. A Gen. 2001, 221, 15–21. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Studer, M. The role of catalysis for the clean production of fine chemicals. Appl. Catal. A Gen. 1999, 189, 191–204. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Costantino, U.; Curini, M.; Montanari, F.; Nocchetti, M.; Rosati, O. Hydrotalcite-like compounds as catalysts in liquid phase organic synthesis: I. Knoevenagel condensation promoted by [Ni0.73Al0.27(OH)2](CO3)0.135. J. Mol. Catal. A Chem. 2003, 195, 245–252. [Google Scholar] [CrossRef]
- Mantilla, A.; Tzompantzi, F.; Manríquez, M.; Mendoza, G.; Fernández, J.L.; Gómez, R. ZnAlFe mixed oxides obtained from LDH type materials as basic catalyst for the gas phase acetone condensation. Adv. Mat. Res. 2010, 132, 55–60. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G., Jr.; Mo, X. Transesterification of poultry fat with methanol using Mg–Al hydrotalcite derived catalysts. Appl. Catal. A Gen. 2007, 331, 138–148. [Google Scholar] [CrossRef]
- Pavel, O.D.; Bîrjega, R.; Che, M.; Costentin, G.; Angelescu, E.; Şerban, S. The activity of Mg/Al reconstructed hydrotalcites by “memory effect” in the cyanoethylation reaction. Catal. Commun. 2008, 9, 1974–1978. [Google Scholar] [CrossRef]
- Pavel, O.D.; Cojocaru, B.; Angelescu, E.; Pârvulescu, V.I. The activity of yttrium-modified Mg, Al hydrotalcites in the epoxidation of styrene with hydrogen peroxide. Appl. Catal. A Gen. 2011, 403, 83–90. [Google Scholar] [CrossRef]
- Lavikainen, L.P.; Hirvi, J.T.; Kasa, S.; Pakkanen, T.A. Interaction of octahedral Mg(II) and tetrahedral Al(III) substitutions in aluminium-rich dioctahedral smectites. Theor. Chem. Acc. 2016, 135, 85. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. Sci. Eng. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Miquel, S.; Primo, J. Catalysts for the production of fine chemicals: Production of food emulsifiers, monoglycerides, by glycerolysis of fats with solid base catalysts. J. Catal. 1998, 173, 315–321. [Google Scholar] [CrossRef]
- Ono, Y. Solid base catalysts for the synthesis of fine chemicals. J. Catal. 2003, 216, 406–415. [Google Scholar] [CrossRef]
- Zhang, F.; Xiang, X.; Li, F.; Duan, X. Layered double hydroxides as catalytic materials: Recent development. Catal. Surv. Asia 2008, 12, 253–265. [Google Scholar] [CrossRef]
- Tichit, D.; Lutic, D.; Coq, B.; Durand, R.; Teissier, R. The aldol condensation of acetaldehyde and heptanal on hydrotalcite-type catalysts. J. Catal. 2003, 219, 167–175. [Google Scholar] [CrossRef]
- Jiao, N.; Stahl, S.S. (Eds.) Green Oxidation in Organic Synthesis; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Angelescu, E.; Ionescu, R.; Pavel, O.D.; Zăvoianu, R.; Bîrjega, R.; Luculescu, C.R.; Florea, M.; Olar, R. Epoxidation of cyclohexene with O2 and isobutyraldehyde catalysed by cobalt modified hydrotalcites. J. Mol. Catal. A Chem. 2010, 315, 178–186. [Google Scholar] [CrossRef]
- Litter, M.I.; Candal, R.J.; Meichtry, J.M. (Eds.) Advanced Oxidation Technologies Sustainable Solutions for Environmental Treatments; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781138072886. [Google Scholar]
- Philip, R.M.; Radhika, S.; Abdulla, C.M.A.; Anilkumar, G. Recent trends and prospects in homogeneous manganese-catalysed epoxidation. Adv. Synth. Catal. 2021, 363, 1272–1289. [Google Scholar] [CrossRef]
- Dusi, M.; Mallat, T.; Baiker, A. Epoxidation of functionalized olefins over solid catalysts. Catal. Rev. Sci. Eng. 2007, 42, 213–278. [Google Scholar] [CrossRef]
- Hauser, S.A.; Cokoja, M.; Kühn, F.E. Epoxidation of olefins with homogeneous catalysts–Quo vadis? Catal. Sci. Technol. 2013, 3, 552–561. [Google Scholar] [CrossRef]
- Ouidri, S.; Guillard, C.; Caps, V.; Khalaf, H. Epoxidation of olefins on photoirradiated TiO2-pillared clays. Appl. Clay Sci. 2010, 48, 431–437. [Google Scholar] [CrossRef]
- Cai, X.; Wang, H.; Zhang, Q.; Tong, J.; Lei, Z. Magnetically recyclable core–shell Fe3O4@chitosan-Schiff base complexes as efficient catalysts for aerobic oxidation of cyclohexene under mild conditions. J. Mol. Catal. A Chem. 2014, 383–384, 217–224. [Google Scholar] [CrossRef]
- El-Korso, S.; Khaldi, I.; Bedrane, S.; Choukchou-Braham, A.; Thibault-Starzyk, F.; Bachir, R. Liquid phase cyclohexene oxidation over vanadia based catalysts with tert-butyl hydroperoxide: Epoxidation versus allylic oxidation. J. Mol. Catal. A Chem. 2014, 394, 89–96. [Google Scholar] [CrossRef]
- Alfayate, A.; Márquez-Álvarez, C.; Grande-Casas, M.; Sánchez-Sánchez, M.; Pérez-Pariente, J. Ti(III)APO-5 materials as selective catalysts for the allylic oxidation of cyclohexene: Effect of Ti source and Ti content. Catal. Today 2014, 227, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Habibia, D.; Faraji, A.R.; Arshadi, M.; Fierro, J.L.G. Characterization and catalytic activity of a novel Fe nano-catalyst as efficient heterogeneous catalyst for selective oxidation of ethylbenzene, cyclohexene, and benzylalcohol. J. Mol. Catal. A Chem. 2013, 372, 90–99. [Google Scholar] [CrossRef]
- Bujak, P.; Bartczak, P.; Polanski, J. Highly efficient room-temperature oxidation of cyclohexene and d-glucose over nanogold Au/SiO2 in water. J. Catal. 2012, 295, 15–21. [Google Scholar] [CrossRef]
- Khare, S.; Chokhare, R. Synthesis, characterization and catalytic activity of Fe(Salen) intercalated α-zirconium phosphate for the oxidation of cyclohexene. J. Mol. Catal. A Chem. 2011, 344, 83–92. [Google Scholar] [CrossRef]
- Chagas, P.; Oliveira, H.S.; Mambrini, R.; Le Hyaric, M.; de Almeida, M.V.; Oliveira, L.C.A. A novel hydrofobic niobium oxyhydroxide as catalyst: Selective cyclohexene oxidation to epoxide. Appl. Catal. A Gen. 2013, 454, 88–92. [Google Scholar] [CrossRef]
- Payne, G.B. Reactions of Hydrogen Peroxide. VII. Alkali-catalyzed epoxidation and oxidation using a nitrile as co-reactant. J. Org. Chem. 1961, 26, 659–663. [Google Scholar] [CrossRef]
- Kirm, I.; Medina, F.; Rodriguez, X.; Cesteros, Y.; Salagre, P.; Sueiras, J. Epoxidation of styrene with hydrogen peroxide using hydrotalcites as heterogeneous catalysts. Appl. Catal. A Gen. 2004, 272, 175–185. [Google Scholar] [CrossRef]
- Mureşeanu, M.; Georgescu, I.; Bibire, L.E.; Cârjă, G. CUII(Sal-Ala)/MgAlLDH and CUII(Sal-Phen)/MgAlLDH as novel catalytic systems for cyclohexene oxidation by H2O2. Catal. Commun. 2014, 54, 39–44. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Bȋrjega, R.; Pavel, O.D.; Cruceanu, A.; Alifanti, M. Hydrotalcite like compounds with low Mo-loading active catalysts for selective oxidation of cyclohexene with hydrogen peroxide. Appl. Catal. A Gen. 2005, 286, 211–220. [Google Scholar] [CrossRef]
- Angelescu, E.; Pavel, O.D.; Bȋrjega, R.; Florea, M.; Zăvoianu, R. The impact of the “memory effect” on the catalytic activity of Mg/Al; Mg,Zn/Al; Mg/Al,Ga hydrotalcite-like compounds used as catalysts for cycloxene epoxidation. Appl. Catal. A Gen. 2008, 341, 50–57. [Google Scholar] [CrossRef]
- Palomeque, J.; Figueras, F.; Gelbard, G. Epoxidation with hydrotalcite-intercalated organotungstic complexes. Appl. Catal. A Gen. 2006, 300, 100–108. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Ionescu, R.; Pavel, O.D.; Bîrjega, R.; Angelescu, E. Comparison between MeIIMg/Al hydrotalcites and hydrotalcite-supported Me(II) acetylacetonates (Me(II) = Co, Cu or Ni) catalysts for the epoxidation of cyclohexene with molecular oxygen. Appl. Clay Sci. 2011, 52, 1–10. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Anderson, J.A. Comparison of the epoxidation of cyclohexene, dicyclopentadiene and 1,5-cyclooctadiene over LDH hosted Fe and Mn sulfonato-salen complexes. J. Mol. Catal. A Chem. 2006, 249, 103–110. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, J.; Chen, Y.; Cui, A.; Sun, F.; He, M.; Xu, Z.; Chen, Q. Metallophthalocyanine intercalated layered double hydroxides as an efficient catalyst for the selective epoxidation of olefin with oxygen. Appl. Catal. A Gen. 2017, 542, 191–200. [Google Scholar] [CrossRef]
- Carriazo, D.; Lima, S.; Martín, C.; Pillinger, M.; Valente, A.A.; Rives, V. Metatungstate and tungstoniobate-containing LDHs: Preparation, characterisation and activity in epoxidation of cyclooctene. J. Phys. Chem. Solids 2007, 68, 1872–1880. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; De Witte, K.; Van Tendeloo, G.; Cool, P.; Vansant, E.F. Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater. 2008, 113, 296–304. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Mantilla, A.; Banũelos, F.; Fernández, J.L.; Gómez, R. Improved photocatalytic degradation of phenolic compounds with znal mixed oxides obtained from LDH Materials. Top. Catal. 2011, 54, 257–263. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Scholz, F.; Kahlert, H. The calculation of the solubility of metal hydroxides, oxide-hydroxides, and oxides, and their visualisation in logarithmic diagrams. ChemTexts 2015, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Moezzi, A.; Lee, P.-S.; McDonagh, A.M.; Cortie, M.B. On the thermal decomposition of zinc hydroxide nitrate, Zn5(OH)8(NO3)2⋅2H2O. J. Solid State Chem. 2020, 286, 121311. [Google Scholar] [CrossRef]
- Angelescu, E.; Pavel, O.D.; Zavoianu, R.; Birjega, R. Cyanoethylation of ethanol over mixed oxides obtained from hydrotalcite, precursors. Rev. Roum. Chim. 2004, 49, 367–375. [Google Scholar]
- Starukh, G.; Rozovik, O.; Oranska, O. Organo/Zn-Al LDH nanocomposites for cationic dye removal from aqueous media. Nanoscale Res. Lett. 2016, 11, 228. [Google Scholar] [CrossRef] [Green Version]
- Navajas, A.; Arzamendi, G.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A.; Gandía, L.M. DRIFTS study of methanol adsorption on Mg–Al hydrotalcite catalysts for the transesterification of vegetable oils. Catal. Commun. 2012, 17, 189–193. [Google Scholar] [CrossRef]
- Kocík, J.; Hájek, M.; Tišler, Z.; Strejcová, K.; Velvarská, R.; Bábelová, M. The influence of long-term exposure of Mg–Al mixed oxide at ambient conditions on its transition to hydrotalcite. J. Solid State Chem. 2021, 304, 122556. [Google Scholar] [CrossRef]
- Yi, H.; Zhao, S.; Tang, X.; Ning, P.; Wang, H.; He, D. Influence of calcination temperature on the hydrolysis of carbonyl sulfide over hydrotalcite-derived Zn–Ni–Al catalyst. Catal. Commun. 2011, 12, 1492–1495. [Google Scholar] [CrossRef]
- Takehira, K.; Kawabata, T.; Shishido, T.; Murakami, K.; Ohi, T.; Shoro, D.; Honda, M.; Takaki, K. Mechanism of reconstitution of hydrotalcite leading to eggshell-type Ni loading on Mg single bondAl mixed oxide. J. Catal. 2005, 231, 92–104. [Google Scholar] [CrossRef]
- Palomeque, J.; Lopez, J.; Figueras, F. Epoxydation of activated olefins by solid bases. J. Catal. 2002, 211, 150–156. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Fornes, V.; Rey, F. Hydrotalcites as Base Catalysts: Influence of the Chemical Composition and Synthesis Conditions on the Dehydrogenation of Isopropanol. J. Catal. 1994, 148, 205–212. [Google Scholar] [CrossRef]
- Jyothi, T.M.; Raja, T.; Sreekumar, K.; Talawar, M.B.; Rao, B.S. Influence of acid–Base properties of mixed oxides derived from hydrotalcite-like precursors in the transfer hydrogenation of propiophenone. J. Mol. Catal. A Chem. 2000, 157, 193–198. [Google Scholar] [CrossRef]
- Debecker, D.; Gaigneaux, E.M.; Busca, G. Exploring, tuning, and exploiting the basicity of hydrotalcites for applications in heterogeneous catalysis. Chem. Eur. J. 2009, 15, 3920–3935. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.; Das, J. Mg/Al hydrotalcites: Preparation, characterisation and ketonisation of acetic acid. J. Mol. Catal. A Chem. 2000, 151, 185–192. [Google Scholar] [CrossRef]
- Ionescu, R.; Pavel, O.D.; Bîrjega, R.; Zăvoianu, R.; Angelescu, E. Epoxidation of cyclohexene with H2O2 and acetonitrile catalyzed by Mg–Al hydrotalcite and cobalt modified hydrotalcites. Catal. Lett. 2010, 134, 309–317. [Google Scholar] [CrossRef]
- Pavel, O.D.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E. The effect of ageing step elimination on the memory effect presented by Mg0.75Al0.25 hydrotalcites (HT) and their catalytic activity for cyanoethylation reaction. Catal. Commun. 2011, 12, 845–850. [Google Scholar] [CrossRef]












| Samples | The Composition Percent (% Mass) | Mg/Al Molar Ratio | Zn/Al Molar Ratio | CO32−/Al Molar Ratio | H2O/Al Molar Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mg | Zn | Al | CO32− | NO3− | H2O | |||||
| HT Mg2Al | 19.44 | n.d. | 11.19 | 14.17 | n.d. | 17.98 | 1.93 | - | 0.57 | 2.41 |
| HT Mg2.5Al | 21.07 | n.d. | 9.55 | 11.68 | n.d. | 15.60 | 2.45 | - | 0.55 | 2.45 |
| HT Mg3Al | 22.64 | n.d. | 8.98 | 10.98 | n.d. | 14.97 | 2.80 | - | 0.55 | 2.50 |
| HT Mg4Al | 24.86 | n.d. | 7.46 | 8.79 | n.d. | 14.98 | 3.70 | - | 0.53 | 3.01 |
| HT Mg5Al | 27.64 | n.d. | 6.40 | 7.39 | n.d. | 11.99 | 4.80 | - | 0.52 | 2.81 |
| HT Zn2Al | n.d. | 37.83 | 8.87 | 10.25 | 0.82 | 12.42 | - | 1.76 | 0.52 | 2.10 |
| HT Zn2.5Al | n.d. | 40.44 | 7.98 | 9.01 | 0.91 | 11.66 | - | 2.10 | 0.51 | 2.20 |
| HT Zn3Al | n.d. | 43.20 | 7.08 | 8.34 | 2.12 | 10.29 | - | 2.52 | 0.53 | 2.18 |
| HT Zn4Al | n.d. | 46.65 | 6.13 | 7.02 | 3.25 | 8.34 | - | 3.14 | 0.52 | 2.04 |
| HT Zn5Al | n.d. | 50.75 | 4.81 | 5.87 | 4.48 | 6.44 | - | 4.36 | 0.55 | 2.01 |
| HT MgZn3Al | 4.92 | 40.12 | 5.56 | 6.55 | n.d. | 10.24 | 0.98 | 2.98 | 0.53 | 2.76 |
| HT Mg2Zn2Al | 11.10 | 30.21 | 6.01 | 6.92 | n.d. | 10.55 | 2.05 | 2.07 | 0.52 | 2.63 |
| HT Mg3ZnAl | 18.10 | 16.02 | 6.67 | 7.61 | n.d. | 11.03 | 3.01 | 0.99 | 0.51 | 2.48 |
| Sample | Ssp (m2∙g−1) | Pore Volume (cm3·g−1) | Pore Radius (Å) | Total Basicity (mmol AA·g−1) | Strong Basic Sites (mmol PhOH·g−1) | Weak + Medium Basic Sites * (mmol·g−1) |
|---|---|---|---|---|---|---|
| HT Mg2Al | 141 | 0.734 | 207 | 4.05 | 0.25 | 3.80 |
| HT Mg2.5Al | 144 | 0.741 | 204 | 4.92 | 0.32 | 4.60 |
| HT Mg3Al | 89 | 0.619 | 251 | 6.56 | 0.26 | 6.30 |
| HT Mg4Al | 84 | 0.592 | 260 | 6.95 | 0.35 | 6.60 |
| HT Mg5Al | 52 | 0.418 | 188 | 7.00 | 0.10 | 6.90 |
| HT Zn2Al | 103 | 0.650 | 239 | 3.65 | 0.15 | 3.50 |
| HT Zn2.5Al | 110 | 0.665 | 233 | 3.94 | 0.24 | 3.70 |
| HT Zn3Al | 83 | 0.606 | 256 | 4.70 | 0.30 | 4.40 |
| HT Zn4Al | 75 | 0.549 | 273 | 5.47 | 0.37 | 5.10 |
| HT Zn5Al | 43 | 0.517 | 290 | 6.42 | 0.42 | 6.00 |
| HT Mg3ZnAl | 136 | 0.723 | 211 | 7.25 | 0.50 | 6.75 |
| HT Mg2Zn2Al | 102 | 0.648 | 240 | 6.54 | 0.44 | 6.10 |
| HT MgZn3Al | 76 | 0.588 | 263 | 5.60 | 0.40 | 5.20 |
| Sample | Ssp (m2·g−1) | Pore Volume (cm3·g−1) | Pore Radius (Å) | Total Basicity (mmol AA·g−1) | Strong Basic Sites (mmol PhOH·g−1) | Weak + Medium Basic Sites * (mmol·g−1) |
|---|---|---|---|---|---|---|
| CHT Mg2Al | 192 | 0.518 | 108 | 6.12 | 0.16 | 5.96 |
| CHT Mg2.5Al | 196 | 0.646 | 110 | 8.10 | 0.24 | 7.86 |
| CHT Mg3Al | 188 | 0.475 | 150 | 8.36 | 0.38 | 7.98 |
| CHT Mg4Al | 230 | 0.754 | 120 | 9.17 | 0.47 | 8.70 |
| CHT Mg5Al | 198 | 0.653 | 109 | 11.43 | 0.78 | 10.65 |
| CHT Zn2Al | 140 | 0.732 | 206 | 4.88 | 0.22 | 4.66 |
| CHT Zn2.5Al | 150 | 0.784 | 192 | 6.41 | 0.39 | 6.02 |
| CHT Zn3Al | 132 | 0.433 | 209 | 7.40 | 0.40 | 7.00 |
| CHT Zn4Al | 202 | 0.662 | 137 | 8.59 | 0.42 | 8.17 |
| CHT Zn5Al | 164 | 0.537 | 169 | 10.50 | 0.70 | 9.80 |
| CHT Mg3ZnAl | 262 | 0.859 | 105 | 10.50 | 0.51 | 9.99 |
| CHT Mg2Zn2Al | 248 | 0.813 | 118 | 9.04 | 0.44 | 8.60 |
| CHT MgZn3Al | 238 | 0.78 | 116 | 8.64 | 0.43 | 8.21 |
| Sample | Ssp (m2·g−1) | Pore Volume (cm3·g−1) | Pore Radius (Å) | Total Basicity (mmol AA·g−1) | Strong Basic Sites (mmol PhOH·g−1) | Weak + Medium Basic Sites * (mmol·g−1) |
|---|---|---|---|---|---|---|
| RHT Mg2Al | 15 | 0.117 | 285 | 4.87 | 0.17 | 4.70 |
| RHT Mg2.5Al | 26 | 0.183 | 236 | 5.96 | 0.26 | 5.70 |
| RHT Mg3Al | 7 | 0.025 | 640 | 7.21 | 0.34 | 6.87 |
| RHT Mg4Al | 5 | 0.018 | 895 | 7.42 | 0.52 | 6.90 |
| RHT Mg5Al | 18 | 0.141 | 238 | 8.47 | 0.57 | 7.90 |
| RHT Zn2Al | 21 | 0.164 | 204 | 4.68 | 0.18 | 4.50 |
| RHT Zn2.5Al | 39 | 0.239 | 196 | 5.64 | 0.24 | 5.40 |
| RHT Zn3Al | 42 | 0.257 | 182 | 5.80 | 0.30 | 5.50 |
| RHT Zn4Al | 18 | 0.149 | 209 | 6.22 | 0.42 | 5.80 |
| RHT Zn5Al | 19 | 0.154 | 208 | 7.11 | 0.51 | 6.60 |
| RHT Mg3ZnAl | 28 | 0.197 | 219 | 8.78 | 0.58 | 8.20 |
| RHT Mg2Zn2Al | 42 | 0.296 | 146 | 8.25 | 0.55 | 7.70 |
| RHT MgZn3Al | 34 | 0.239 | 180 | 8.04 | 0.54 | 7.50 |
| Catalyst | Yield to Epoxycyclohexane After 5 h (%) | ||||
|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | |
| CHT Mg5Al | 99.8 | 89.8 | 84.3 | 79.2 | 75.0 |
| CHT Mg4Al | 95.0 | 82.0 | 76.3 | 69.7 | 64.0 |
| CHT Zn5Al | 97.7 | 93.6 | 89.2 | 87.5 | 85.2 |
| CHT Mg3ZnAl | 98.5 | 95.3 | 92.4 | 90.5 | 88.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zăvoianu, R.; Cruceanu, A.; Pavel, O.D.; Bradu, C.; Florea, M.; Bîrjega, R. Green Epoxidation of Olefins with ZnxAl/MgxAl-LDH Compounds: Influence of the Chemical Composition. Catalysts 2022, 12, 145. https://doi.org/10.3390/catal12020145
Zăvoianu R, Cruceanu A, Pavel OD, Bradu C, Florea M, Bîrjega R. Green Epoxidation of Olefins with ZnxAl/MgxAl-LDH Compounds: Influence of the Chemical Composition. Catalysts. 2022; 12(2):145. https://doi.org/10.3390/catal12020145
Chicago/Turabian StyleZăvoianu, Rodica, Anca Cruceanu, Octavian Dumitru Pavel, Corina Bradu, Mihaela Florea, and Ruxandra Bîrjega. 2022. "Green Epoxidation of Olefins with ZnxAl/MgxAl-LDH Compounds: Influence of the Chemical Composition" Catalysts 12, no. 2: 145. https://doi.org/10.3390/catal12020145
APA StyleZăvoianu, R., Cruceanu, A., Pavel, O. D., Bradu, C., Florea, M., & Bîrjega, R. (2022). Green Epoxidation of Olefins with ZnxAl/MgxAl-LDH Compounds: Influence of the Chemical Composition. Catalysts, 12(2), 145. https://doi.org/10.3390/catal12020145