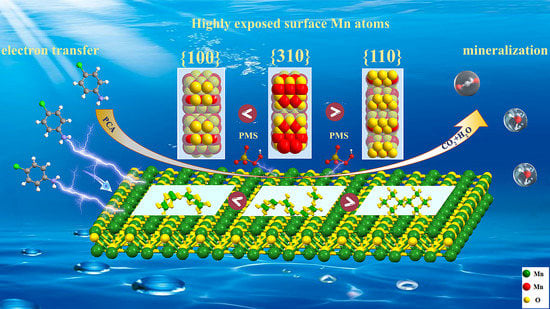
Regulating Crystal Facets of MnO2 for Enhancing Peroxymonosulfate Activation to Degrade Pollutants: Performance and Mechanism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalyst
2.2. PCA Degradation in the Facet-Engineered MnO2/PMS System
2.2.1. PCA Degradation in Different α-MnO2/PMS Systems
2.2.2. Effect of PMS Concentration
2.2.3. Effect of Catalyst Dosage
2.2.4. Effect of Initial pH
2.3. Stability and Universal Adaptability
2.3.1. Universal Adaptability of 310-MnO2
2.3.2. Stability of 310-MnO2
2.4. Mechanism of PMS Activation by 310-MnO2
2.4.1. Quenching Experiments and PMS Decomposition Experiments
2.4.2. EPR Detection and Solvent Exchange Experiment
2.4.3. Electrochemical Analysis and Sulfhydryl Modification Experiments
2.4.4. DFT Calculations
2.5. Removal of TOC and Proposed Pathway of PCA Degradation
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of α-MnO2 with Different Facets
3.3. Experimental Procedures
3.4. Analytical Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, A.; Podkoscielny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.; Park, C.M.; Jang, M.; Kim, D.-H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Haritash, A.; Kaushik, C. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, H.; Yang, C.; Li, X.; Lin, Y.; Yin, K.; Sun, J.; Teng, Q.; Du, C.; Zhong, Y. High-performance porous carbon catalysts doped by iron and nitrogen for degradation of bisphenol F via peroxymonosulfate activation. Chem. Eng. J. 2019, 392, 123683. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Zhu, Z.; Cui, F.; Zhu, Y.-A.; Duan, X.; Wang, S. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Chen, T.; Yang, N.; Wang, S.; Ding, X.; Chen, H. Deep insight into ROS mediated direct and hydroxylated dichlorination process for efficient photocatalytic sodium pentachlorophenate mineralization. Appl. Catal. B Environ. 2021, 296, 120352. [Google Scholar] [CrossRef]
- Tay, K.S.; Madehi, N. Ozonation of ofloxacin in water: By-products, degradation pathway and ecotoxicity assessment. Sci. Total Environ. 2015, 520, 23–31. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Aziz, K.H.H. Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor. Chemosphere 2019, 228, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, Q.; Wang, J.; Chen, Z.; Chen, Z. Nonradical oxidation processes in PMS-based heterogeneous catalytic system: Generation, identification, oxidation characteristics, challenges response and application prospects. Chem. Eng. J. 2020, 410, 128312. [Google Scholar] [CrossRef]
- Wu, L.; Li, B.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Xia, Q.; Peng, W. Preferential Growth of the Cobalt (200) Facet in Co@N–C for Enhanced Performance in a Fenton-like Reaction. ACS Catal. 2021, 11, 5532–5543. [Google Scholar] [CrossRef]
- He, D.; Li, Y.; Lyu, C.; Song, L.; Feng, W.; Zhang, S. New insights into MnOOH/peroxymonosulfate system for catalytic oxidation of 2,4-dichlorophenol: Morphology dependence and mechanisms. Chemosphere 2020, 255, 126961. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Singewald, K.; Liu, C.-C.; Saxena, S.; Zhang, H. Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions. Chem. Eng. J. 2019, 370, 906–915. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B: Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Huang, K.Z.; Zhang, H.J. Direct Electron-Transfer-Based Peroxymonosulfate Activation by Iron-Doped Manganese Oxide (δ-MnO2) and the Development of Galvanic Oxidation Processes (GOPs). Environ. Sci. Technol. 2019, 53, 12610–12620. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Liu, Z.; Peng, Z.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Greatly enhanced oxidative activity of δ-MnO2 to degrade organic pollutants driven by dominantly exposed {−111} facets. J. Hazard. Mater. 2021, 413, 125285. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, J.; Duan, X.; Tan, X.; Liu, S.; Wang, S. Self-assembly of 3D MnO2/N-doped graphene hybrid aerogel for catalytic degradation of water pollutants: Structure-dependent activity. Chem. Eng. J. 2019, 369, 1049–1058. [Google Scholar] [CrossRef]
- Yu, J.; Zeng, T.; Wang, H.; Zhang, H.; Sun, Y.; Chen, L.; Song, S.; Li, L.; Shi, H. Oxygen-defective MnO2−x rattle-type microspheres mediated singlet oxygen oxidation of organics by peroxymonosulfate activation. Chem. Eng. J. 2020, 394, 124458. [Google Scholar] [CrossRef]
- He, C.; Wang, Y.; Li, Z.; Huang, Y.; Liao, Y.; Xia, D.; Lee, S.-C. Facet Engineered α-MnO2 for Efficient Catalytic Ozonation of Odor CH3SH: Oxygen Vacancy-Induced Active Centers and Catalytic Mechanism. Environ. Sci. Technol. 2020, 54, 12771–12783. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhu, Y.; Curnan, M.T.; Zhang, Y.; Han, J.W.; Chen, Y.; Huang, S.; Lin, Z. Tuning reaction pathways of peroxymonosulfate-based advanced oxidation process via defect engineering. Cell Rep. Phys. Sci. 2021, 2, 100550. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Si, F.; Yao, C.; Du, M.; Hussain, I.; Kim, H.; Huang, S.; Lin, Z.; Hayat, W. Persulfate non-radical activation by nano-CuO for efficient removal of chlorinated organic compounds: Reduced graphene oxide-assisted and CuO (0 0 1) facet-dependent. Chem. Eng. J. 2018, 356, 178–189. [Google Scholar] [CrossRef]
- Rong, S.; Zhang, P.; Liu, F.; Yang, Y. Engineering Crystal Facet of α-MnO2 Nanowire for Highly Efficient Catalytic Oxidation of Carcinogenic Airborne Formaldehyde. ACS Catal. 2018, 8, 3435–3446. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Wang, Y.; Huang, J.; Wang, W.; Duan, L.; Yang, X.; Yu, X.; Han, X.; Liu, N. Nonradical activation of peroxydisulfate promoted by oxygen vacancy-laden NiO for catalytic phenol oxidative polymerization. Appl. Catal. B Environ. 2019, 254, 166–173. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.-Y.; Sun, S.-G.; Ding, Y.; Wang, Z.L. Synthesis of Tetrahexahedral Platinum Nanocrystals with High-Index Facets and High Electro-Oxidation Activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Wang, F.; Dai, H.; Deng, J.; Bai, G.; Ji, K.; Liu, Y. Manganese Oxides with Rod-, Wire-, Tube-, and Flower-Like Morphologies: Highly Effective Catalysts for the Removal of Toluene. Environ. Sci. Technol. 2012, 46, 4034–4041. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Wang, S.; Deng, S.; Wang, B.; Yu, G. Catalytic removal of gaseous unintentional POPs on manganese oxide octahedral molecular sieves. Appl. Catal. B Environ. 2013, 142–143, 568–578. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, Z.; Ding, Z.; Liu, J. Synthesis of different crystallographic FeOOH catalysts for peroxymonosulfate activation towards organic matter degradation. RSC Adv. 2018, 8, 7269–7279. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Fan, S.; Li, Y.; Zhou, Q. Fe-N/C single-atom catalysts with high density of Fe-Nx sites toward peroxymonosulfate activation for high-efficient oxidation of bisphenol A: Electron-transfer mechanism. Chem. Eng. J. 2021, 419, 129590. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Zhou, S.; Huang, R.; Huang, S.; Kuang, H.; Zeng, X.; Zhao, S. Activation of persulfate by MnOOH: Degradation of organic compounds by nonradical mechanism. Chemosphere 2021, 272, 129629. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Jiang, L.; Wang, K.; Li, Y.; Huang, H.; Wu, X.; Zhang, G. Efficient persulfate activation by hematite nanocrystals for degradation of organic pollutants under visible light irradiation: Facet-dependent catalytic performance and degradation mechanism. Appl. Catal. B Environ. 2021, 286, 119883. [Google Scholar] [CrossRef]
- Chen, X.; Duan, X.; Oh, W.-D.; Zhang, P.-H.; Guan, C.-T.; Zhu, Y.-A.; Lim, T.-T. Insights into nitrogen and boron-co-doped graphene toward high-performance peroxymonosulfate activation: Maneuverable N-B bonding configurations and oxidation pathways. Appl. Catal. B Environ. 2019, 253, 419–432. [Google Scholar] [CrossRef]
- Cao, J.; Lai, L.; Lai, B.; Yao, G.; Chen, X.; Song, L. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chem. Eng. J. 2019, 364, 45–56. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Hussain, I.; Huang, S.; Huang, W. Insight into reactive oxygen species in persulfate activation with copper oxide: Activated persulfate and trace radicals. Chem. Eng. J. 2017, 313, 1023–1032. [Google Scholar] [CrossRef]
- Lutze, H.V.; Kerlin, N.; Schmidt, T.C. Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res. 2015, 72, 349–360. [Google Scholar] [CrossRef]
- Bennedsen, L.R.; Muff, J.; Søgaard, E.G. Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere 2012, 86, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation: Kinetics, reaction products and transformation mechanisms. J. Hazard. Mater. 2015, 285, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Chu, W.; Xu, B. Modeling the heterogeneous peroxymonosulfate/Co-MCM41 process for the degradation of caffeine and the study of influence of cobalt sources. Chem. Eng. J. 2013, 235, 10–18. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croué, J.-P. Production of Sulfate Radical from Peroxymonosulfate Induced by a Magnetically Separable CuFe2O4 Spinel in Water: Efficiency, Stability, and Mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.; Wang, Y.; Le Roux, J.; Yang, Y.; Croué, J.-P. Efficient Peroxydisulfate Activation Process Not Relying on Sulfate Radical Generation for Water Pollutant Degradation. Environ. Sci. Technol. 2014, 48, 5868–5875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, W.; Zhang, Y.; Hussain, I.; Huang, S.; Du, X. Comparison of radical and non-radical activated persulfate systems for the degradation of imidacloprid in water. Ecotoxicol. Environ. Saf. 2019, 188, 109891. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-I.; Weon, S.; Choi, W.; Hwang, Y.S.; Seo, J.; Lee, C.; Kim, J.-H. Activation of Persulfates by Graphitized Nanodiamonds for Removal of Organic Compounds. Environ. Sci. Technol. 2016, 50, 10134–10142. [Google Scholar] [CrossRef]
- Chen, J.; Fang, C.; Xia, W.; Huang, T.; Huang, C.-H. Selective Transformation of β-Lactam Antibiotics by Peroxymonosulfate: Reaction Kinetics and Nonradical Mechanism. Environ. Sci. Technol. 2018, 52, 1461–1470. [Google Scholar] [CrossRef]
- Yun, E.-T.; Lee, J.H.; Kim, J.; Park, H.-D.; Lee, J. Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation Versus Mediated Electron Transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef]
- Qin, J.; Dai, L.; Shi, P.; Fan, J.; Min, Y.; Xu, Q. Rational design of efficient metal-free catalysts for peroxymonosulfate activation: Selective degradation of organic contaminants via a dual nonradical reaction pathway. J. Hazard. Mater. 2020, 398, 122808. [Google Scholar] [CrossRef]
- Yuan, R.; Jiang, M.; Gao, S.; Wang, Z.; Wang, H.; Boczkaj, G.; Liu, Z.; Ma, J.; Li, Z. 3D mesoporous α-Co(OH)2 nanosheets electrodeposited on nickel foam: A new generation of macroscopic cobalt-based hybrid for peroxymonosulfate activation. Chem. Eng. J. 2019, 380, 122447. [Google Scholar] [CrossRef]
- Huang, G.; Chuan-Wang, Y.; Yang, C.-W.; Guo, P.-C.; Yu, H.-Q. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn1.8Fe1.2O4 Nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ji, Z.-Y.; Zhao, Y.-Y.; Liu, J.; Li, F.; Yuan, J.-S. Treatment of wastewater containing 2-methoxyphenol by persulfate with thermal and alkali synergistic activation: Kinetics and mechanism. Chem. Eng. J. 2019, 380, 122411. [Google Scholar] [CrossRef]
- Yang, S.; Wu, P.; Liu, J.; Chen, M.; Ahmed, Z.; Zhu, N. Efficient removal of bisphenol A by superoxide radical and singlet oxygen generated from peroxymonosulfate activated with Fe0-montmorillonite. Chem. Eng. J. 2018, 350, 484–495. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, P.-H.; Wu, D.; Shih, K. Surface-bound sulfate radical-dominated degradation of 1,4-dioxane by alumina-supported palladium (Pd/Al2O3) catalyzed peroxymonosulfate. Water Res. 2017, 120, 12–21. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. J. Hazard. Mater. 2019, 384, 121350. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Qian, X.; Chen, L.; Cai, T. Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant. Appl. Catal. B Environ. 2019, 263, 118348. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, Y.; Kang, J.; Wang, S. N-Doping-Induced Nonradical Reaction on Single-Walled Carbon Nanotubes for Catalytic Phenol Oxidation. ACS Catal. 2014, 5, 553–559. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate Activation on Crystallographic Manganese Oxides: Mechanism of Singlet Oxygen Evolution for Nonradical Selective Degradation of Aqueous Contaminants. Environ. Sci. Technol. 2018, 53, 307–315. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Xiao, F.; Huang, R.; Gao, S.; Cui, F.; Wang, S.; Duan, X. Structure-dependent catalysis of cuprous oxides in peroxymonosulfate activation via nonradical pathway with a high oxidation capacity. J. Hazard. Mater. 2019, 385, 121518. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Jiang, J.; Pang, S.; Luo, C.; Ma, J.; Zhou, Y.; Yang, Y. Oxidation Kinetics of Bromophenols by Nonradical Activation of Peroxydisulfate in the Presence of Carbon Nanotube and Formation of Brominated Polymeric Products. Environ. Sci. Technol. 2017, 51, 10718–10728. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.J.; Yao, N.; Charnock, F.T.; Kennedy, T.A. High-Quality Manganese-Doped ZnSe Nanocrystals. Nano Lett. 2000, 1, 3–7. [Google Scholar] [CrossRef]
- Gorman, A.A.; Rodgers, M.A.J. Singlet molecular oxygen. Chem. Soc. Rev. 1981, 10, 205–231. [Google Scholar] [CrossRef]
- Nardi, G.; Manet, I.; Monti, S.; Miranda, M.A.; Lhiaubet-Vallet, V. Scope and limitations of the TEMPO/EPR method for singlet oxygen detection: The misleading role of electron transfer. Free Radic. Biol. Med. 2014, 77, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.-Y.; Li, J.; Lu, X.-T.; Yuan, L.-P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef]
- Wang, M.; Cui, Y.; Cao, H.; Wei, P.; Chen, C.; Li, X.; Xu, J.; Sheng, G. Activating peroxydisulfate with Co3O4/NiCo2O4 double-shelled nanocages to selectively degrade bisphenol A—A nonradical oxidation process. Appl. Catal. B Environ. 2020, 282, 119585. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, Y.; Du, M.; Du, X.; Huang, S. Insights into the mechanism of non-radical activation of persulfate via activated carbon for the degradation of p-chloroaniline. Chem. Eng. J. 2019, 362, 262–268. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.-F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Gao, P.; Wang, L.; Zhang, Y.; Deng, Y.; Huang, R.; Zhao, S.; Yu, Z.; Wei, Y.; Wang, G.; et al. Regulating Crystal Facets of MnO2 for Enhancing Peroxymonosulfate Activation to Degrade Pollutants: Performance and Mechanism. Catalysts 2022, 12, 342. https://doi.org/10.3390/catal12030342
Fu J, Gao P, Wang L, Zhang Y, Deng Y, Huang R, Zhao S, Yu Z, Wei Y, Wang G, et al. Regulating Crystal Facets of MnO2 for Enhancing Peroxymonosulfate Activation to Degrade Pollutants: Performance and Mechanism. Catalysts. 2022; 12(3):342. https://doi.org/10.3390/catal12030342
Chicago/Turabian StyleFu, Juncong, Peng Gao, Lu Wang, Yongqing Zhang, Yuhui Deng, Renfeng Huang, Shuaifei Zhao, Zebin Yu, Yuancheng Wei, Guangzhao Wang, and et al. 2022. "Regulating Crystal Facets of MnO2 for Enhancing Peroxymonosulfate Activation to Degrade Pollutants: Performance and Mechanism" Catalysts 12, no. 3: 342. https://doi.org/10.3390/catal12030342
APA StyleFu, J., Gao, P., Wang, L., Zhang, Y., Deng, Y., Huang, R., Zhao, S., Yu, Z., Wei, Y., Wang, G., & Zhou, S. (2022). Regulating Crystal Facets of MnO2 for Enhancing Peroxymonosulfate Activation to Degrade Pollutants: Performance and Mechanism. Catalysts, 12(3), 342. https://doi.org/10.3390/catal12030342