Sm-CeO2/Zeolite Bifunctional Catalyst for Direct and Highly Selective Conversion of Bioethanol to Propylene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Textural Properties
2.2. NH3-TPD and CO2-TPD
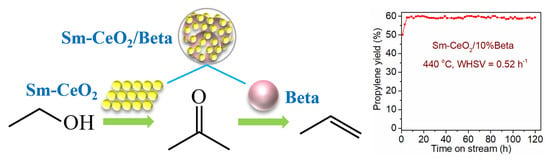
2.3. Catalytic Performances
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization of Catalyst
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Wang, Y. Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Blay, V.; Louis, B.; Miravalles, R.; Yokoi, T.; Peccatiello, K.A.; Clough, M.; Yilmaz, B. Engineering zeolites for catalytic cracking to light olefins. ACS Catal. 2017, 7, 6542–6566. [Google Scholar] [CrossRef]
- Song, Z.; Takahashi, A.; Mimura, N.; Fujitani, T. Production of propylene from ethanol over ZSM-5 zeolites. Catal. Lett. 2009, 131, 364–369. [Google Scholar] [CrossRef]
- Goto, D.; Harada, Y.; Furumoto, Y.; Takahashi, A.; Fujitani, T.; Oumi, Y.; Sadakane, M.; Sano, T. Conversion of ethanol to propylene over HZSM-5 type zeolites containing alkaline earth metals. Appl. Catal. A 2010, 383, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Takahashi, A.; Nakamura, I.; Fujitani, T. Phosphorus-modified ZSM-5 for conversion of ethanol to propylene. Appl. Catal. A 2010, 384, 201–205. [Google Scholar] [CrossRef]
- Bi, J.D.; Liu, M.; Song, C.S.; Wang, X.S.; Guo, X.W. C2–C4 light olefins from bioethanol catalyzed by Ce-modified nanocrystalline HZSM-5 zeolite catalysts. Appl. Catal. B 2011, 107, 68–76. [Google Scholar] [CrossRef]
- Furumoto, Y.; Harada, Y.; Tsunoji, N.; Takahashi, A.; Fujitani, T.; Ide, Y.; Sadakane, M.; Sano, T. Effect of acidity of ZSM-5 zeolite on conversion of ethanol to propylene. Appl. Catal. A 2011, 399, 262–267. [Google Scholar] [CrossRef]
- Takahashi, A.; Xia, W.; Nakamura, I.; Shimada, H.; Fujitani, T. Effects of added phosphorus on conversion of ethanol to propylene over ZSM-5 catalysts. Appl. Catal. A 2012, 423, 162–167. [Google Scholar] [CrossRef]
- Meng, T.; Mao, D.S.; Guo, Q.S.; Lu, G.Z. The effect of crystal sizes of HZSM-5 zeolites in ethanol conversion to propylene. Catal. Commun. 2012, 21, 52–57. [Google Scholar] [CrossRef]
- Xia, W.; Chen, K.; Takahashi, A.; Li, X.Y.; Mu, X.C.; Han, C.; Liu, L.; Nakamura, I.; Fujitani, T. Effects of particle size on catalytic conversion of ethanol to propylene over H-ZSM-5 catalysts—Smaller is better. Catal. Commun. 2016, 73, 27–33. [Google Scholar] [CrossRef]
- Huangfu, J.J.; Mao, D.S.; Zhai, X.L.; Guo, Q.S. Remarkably enhanced stability of HZSM-5 zeolite co-modified with alkaline and phosphorous for the selective conversion of bio-ethanol to propylene. Appl. Catal. A 2016, 520, 99–104. [Google Scholar] [CrossRef]
- Zhang, N.; Mao, D.S.; Zhai, X.L. Selective conversion of bio-ethanol to propene over nano-HZSM-5 zeolite: Remarkably enhanced catalytic performance by fluorine modification. Fuel Process. Technol. 2017, 167, 50–60. [Google Scholar] [CrossRef]
- Hayashi, F.; Tanaka, M.; Lin, D.; Iwamoto, M. Surface structure of yttrium-modified ceria catalysts and reaction pathways from ethanol to propene. J. Catal. 2014, 316, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, M.; Mizuno, S.; Tanaka, M. Direct and selective production of propene from bio-ethanol on Sc-loaded In2O3 catalysts. Chem. Eur. J. 2013, 19, 7214–7220. [Google Scholar] [CrossRef]
- Xia, W.; Wang, F.F.; Mu, X.C.; Chen, K. Remarkably enhanced selectivity for conversion of ethanol to propylene over ZrO2 catalysts. Fuel Process. Technol. 2017, 166, 140–145. [Google Scholar] [CrossRef]
- Xia, W.; Wang, F.F.; Mu, X.C.; Chen, K.; Takahashi, A.; Nakamura, I.; Fujitani, T. Highly selective catalytic conversion of ethanol to propylene over yttrium-modified zirconia catalyst. Catal. Commun. 2017, 90, 10–13. [Google Scholar] [CrossRef]
- Xue, F.Q.; Miao, C.X.; Yue, Y.H.; Hua, W.M.; Gao, Z. Direct conversion of bio-ethanol to propylene in high yield over the composite of In2O3 and zeolite beta. Green Chem. 2017, 19, 5582–5590. [Google Scholar] [CrossRef]
- Xue, F.Q.; Miao, C.X.; Yue, Y.H.; Hua, W.M.; Gao, Z. Sc2O3-promoted composite of In2O3 and Beta zeolite for direct conversion of bio-ethanol to propylene. Fuel Process. Technol. 2019, 186, 110–115. [Google Scholar] [CrossRef]
- Xu, L.L.; Zhao, R.R.; Zhang, W.P. One-step high-yield production of renewable propene from bioethanol over composite ZnCeOx oxide and HBeta zeolite with balanced Brönsted/Lewis acidity. Appl. Catal. B 2020, 279, 119389. [Google Scholar] [CrossRef]
- Jansen, J.C.; Creyghton, E.J.; Njo, S.L.; van Koningsveld, H.; van Bekkum, H. On the remarkable behaviour of zeolite Beta in acid catalysis. Catal. Today 1997, 38, 205–212. [Google Scholar] [CrossRef]
- Creyghton, E.J.; Ganeshie, S.D.; Downing, R.S.; van Bekkum, H. Stereoselective Meerwein–Ponndorf–Verley and Oppenauer reactions catalysed by zeolite BEA. J. Mol. Catal. A 1997, 115, 457–472. [Google Scholar] [CrossRef]
- Klomp, D.; Maschmeyer, T.; Hanefeld, U.; Peters, J.A. Mechanism of homogeneously and heterogeneously catalyzed Meerwein−Ponndorf−Verley−Oppenauer reactions for the racemisation of secondary alcohols. Chem. Eur. J. 2004, 10, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Cheralathan, K.K.; Palanichamy, M.; Murugesan, V. Cyclisation of (phenylthio)acetaldehyde diethylacetal in the presence of dealuminated beta zeolites: An unexpected Meerwein–Ponndorf–Verley reduction. Appl. Catal. A 2004, 263, 219–225. [Google Scholar] [CrossRef]
- Matheus, C.R.V.; Chagas, L.H.; Gonzalez, G.G.; Aguiar, E.F.S.; Appel, L.G. Synthesis of propene from ethanol: A mechanistic study. ACS Catal. 2018, 8, 7667–7678. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B.J. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]










| Catalyst | Sm | Ce | SBET | Vmicro | a = b = c |
|---|---|---|---|---|---|
| (wt%) a | (wt%) a | (m2/g) | (cm3/g) b | (nm) c | |
| Sm-CeO2 | 20.2 | 62.2 | 21 | 0 | 0.5420 |
| SmCe/2%Beta | 19.7 | 61.1 | 30 | 0.004 | 0.5420 |
| SmCe/5%Beta | 19.1 | 59.2 | 54 | 0.009 | 0.5420 |
| SmCe/10%Beta | 18.0 | 56.2 | 69 | 0.015 | 0.5420 |
| SmCe/15%Beta | 17.1 | 53.2 | 93 | 0.022 | 0.5419 |
| SmCe/20%Beta | 16.0 | 49.9 | 124 | 0.032 | 0.5419 |
| Beta | - | - | 546 | 0.183 | - |
| Catalyst | NH3-TPD Result (mmol/g) | CO2-TPD Result (mmol/g) | ||||
|---|---|---|---|---|---|---|
| Weak a | Strong b | Total | Weak d | Moderate e | Total | |
| Sm-CeO2 | 0.036 | 0.082 | 0.118 | 0.042 | 0.069 | 0.111 |
| SmCe/2%Beta | 0.053 | 0.110 | 0.163 | 0.070 | 0.080 | 0.150 |
| SmCe/5%Beta | 0.069 | 0.124 | 0.193 | 0.072 | 0.082 | 0.154 |
| SmCe/10%Beta | 0.083 | 0.150 | 0.233 | 0.072 | 0.083 | 0.155 |
| SmCe/15%Beta | 0.113 | 0.186 | 0.299 | 0.080 | 0.085 | 0.165 |
| SmCe/20%Beta | 0.120 | 0.203 | 0.323 | 0.077 | 0.083 | 0.160 |
| Beta c | 0.515 | 0.364 | 0.879 | - | 0.060 | 0.060 |
| Catalyst | Sm-CeO2 | SmCe/10%Beta | Beta | SmCe/10%ZSM-5 | ZSM-5 |
|---|---|---|---|---|---|
| Conv. (%) | 0.5 | 35.9 | 30.0 | 0.6 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Miao, C.; Yue, Y.; Tian, C.; Hua, W.; Gao, Z. Sm-CeO2/Zeolite Bifunctional Catalyst for Direct and Highly Selective Conversion of Bioethanol to Propylene. Catalysts 2022, 12, 407. https://doi.org/10.3390/catal12040407
Jin H, Miao C, Yue Y, Tian C, Hua W, Gao Z. Sm-CeO2/Zeolite Bifunctional Catalyst for Direct and Highly Selective Conversion of Bioethanol to Propylene. Catalysts. 2022; 12(4):407. https://doi.org/10.3390/catal12040407
Chicago/Turabian StyleJin, Huan, Changxi Miao, Yinghong Yue, Chao Tian, Weiming Hua, and Zi Gao. 2022. "Sm-CeO2/Zeolite Bifunctional Catalyst for Direct and Highly Selective Conversion of Bioethanol to Propylene" Catalysts 12, no. 4: 407. https://doi.org/10.3390/catal12040407
APA StyleJin, H., Miao, C., Yue, Y., Tian, C., Hua, W., & Gao, Z. (2022). Sm-CeO2/Zeolite Bifunctional Catalyst for Direct and Highly Selective Conversion of Bioethanol to Propylene. Catalysts, 12(4), 407. https://doi.org/10.3390/catal12040407