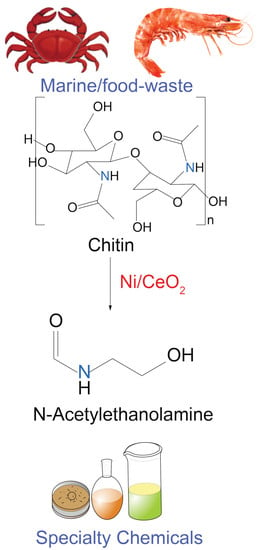
Towards the Efficient Catalytic Valorization of Chitin to N-Acylethanolamine over Ni/CeO2 Catalyst: Exploring the Shape-Selective Reactivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalysis: Chitin Valorization
2.3. Catalysis: Reaction Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CeO2 Supports
3.3. Synthesis of Ni/CeO2 Catalysts
3.4. Instrumentation and Characterization
3.5. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, B. Make recycled goods covetable. Nature 2016, 531, 438–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hülsey, M.J. Shell biorefinery: A comprehensive introduction. Green Energy Environ. 2018, 3, 318–327. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Yan, N. Shell Biorefinery: Dream or Reality? Chemistry 2016, 22, 13402–13421. [Google Scholar] [CrossRef]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, Y.; Zhang, L.; Cai, J. Extremely Strong and Transparent Chitin Films: A High-Efficiency, Energy-Saving, and “Green” Route Using an Aqueous KOH/Urea Solution. Adv. Funct. Mater. 2017, 27, 1701100. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Dai, J.; Li, F.; Fu, X. Towards Shell Biorefinery: Advances in Chemical-Catalytic Conversion of Chitin Biomass to Organonitrogen Chemicals. ChemSusChem. 2020, 13, 6498–6508. [Google Scholar] [CrossRef]
- Pelckmans, M.; Renders, T.; Van de Vyver, S.; Sels, B.F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 2017, 19, 5303–5331. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Luque, R.; Selva, M. Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials. Chem. Soc. Rev. 2020, 49, 4527–4563. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, T.; Kobayashi, H.; Murata, C.; Shichibu, Y.; Konishi, K.; Fukuoka, A. Catalytic Conversion of a Chitin-Derived Sugar Alcohol to an Amide-Containing Isosorbide Analog. ACS Sustain. Chem. Eng. 2019, 7, 14883–14888. [Google Scholar] [CrossRef]
- Sagawa, T.; Kobayashi, H.A. Fukuoka, Effect of Lewis acid on catalytic dehydration of a chitin-derived sugar alcohol. Mol. Catal. 2020, 498, 111282. [Google Scholar] [CrossRef]
- Yabushita, M.; Kobayashi, H.; Kuroki, K.; Ito, S.; Fukuoka, A. Catalytic Depolymerization of Chitin with Retention of N-Acetyl Group. ChemSusChem 2015, 8, 3760–3763. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Techikawara, K.; Fukuoka, A. Hydrolytic hydrogenation of chitin to amino sugar alcohol. Green Chem. 2020, 19, 3350–3356. [Google Scholar] [CrossRef] [Green Version]
- Verduyckt, J.; Coeck, R.; De Vos, D.E. Ru-Catalyzed Hydrogenation–Decarbonylation of Amino Acids to Bio-based Primary Amines. ACS Sustain. Chem. Eng. 2017, 5, 3290–3295. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Zhang, J.; Pierson, Y.; Chen, X.; Yan, N. Conversion of chitin derived N-acetyl-d-glucosamine (NAG) into polyols over transition metal catalysts and hydrogen in water. Green Chem. 2015, 17, 1024–1031. [Google Scholar] [CrossRef]
- Xie, S.; Jia, C.; Go Ong, S.S.; Wang, Z.; Zhu, M.-j.; Wang, Q.; Yang, Y.; Lin, H. A Shortcut Route to Close Nitrogen Cycle: Bio-Based Amines Production via Selective Deoxygenation of Chitin Monomers over Ru/C in Acidic Solutions. iScience 2020, 23, 101096. [Google Scholar] [CrossRef]
- Techikawara, K.; Kobayashi, H.; Fukuoka, A. Conversion of N-Acetylglucosamine to Protected Amino Acid over Ru/C Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 12411–12418. [Google Scholar] [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Zhang, X.; You, R.; Li, D.; Cao, T.; Huang, W. Reaction Sensitivity of Ceria Morphology Effect on Ni/CeO2 Catalysis in Propane Oxidation Reactions. ACS Appl. Mater. Interfaces. 2017, 9, 35897–35907. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.; Pasanen, T.P.; Gädda, A.; Garín, M.; Rosta, K.; Vähänissi, V.; Savin, H. Impact of doping and silicon substrate resistivity on the blistering of atomic-layer-deposited aluminium oxide. Appl. Surf. Sci. 2020, 522, 146400. [Google Scholar] [CrossRef]
- Li, P.; Dong, R.; Jiang, X.; Zhang, S.; Liu, T.; Wang, R.; Yan, F.; Fu, D. The effect of CeO2 morphology on the electrochemical performance of the reversible solid oxide cells. J. Electroanal. Chem. 2020, 873, 114513. [Google Scholar] [CrossRef]
- Chen, D.; Ma, Q.; Wei, L.; Li, N.; Shen, Q.; Tian, W.; Zhou, J.; Long, J. Catalytic hydroliquefaction of rice straw for bio-oil production using Ni/CeO2 catalysts. J. Anal. Appl. Pyrolysis 2018, 130, 169–180. [Google Scholar] [CrossRef]
- Greluk, M.; Gac, W.; Rotko, M.; Słowik, G.; Turczyniak-Surdacka, S. Co/CeO2 and Ni/CeO2 catalysts for ethanol steam reforming: Effect of the cobalt/nickel dispersion on catalysts properties. J. Catal. 2021, 393, 159–178. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Cullen, D.A.; Hu, W.; Huang, J.; Yao, L.; Peng, Z.; Liao, P.; Wang, R. Distribution and Valence State of Ru Species on CeO2 Supports: Support Shape Effect and Its Influence on CO Oxidation. ACS Catal. 2019, 9, 11088–11103. [Google Scholar] [CrossRef]
- Rui, N.; Zhang, X.; Zhang, F.; Liu, Z.; Cao, X.; Xie, Z.; Zou, R.; Senanayake, S.D.; Yang, Y.; Rodriguez, J.A.; et al. Highly active Ni/CeO2 catalyst for CO2 methanation: Preparation and characterization. Appl. Catal. B Environ. 2021, 282, 119581. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Drewett, N.E.; Wang, X.; Yoo, S.J.; Wang, H.; Deng, T.; Kim, J.G.; Chen, H.; Huang, K.; et al. Integrating Catalysis of Methane Decomposition and Electrocatalytic Hydrogen Evolution with Ni/CeO2 for Improved Hydrogen Production Efficiency. ChemSusChem 2019, 12, 1000–1010. [Google Scholar] [CrossRef]
- Liu, B.; Yan, Z.; Xu, T.; Wang, J.; Li, C.; Gao, R.; Bai, J. Promoting electron transfer of surface oxygen vacancies in Pd/CeO2-RE via doping engineering for enhancing catalytic activity in Suzuki coupling reaction. J. Catal. 2021, 399, 15–23. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Wang, Y.; Younesi, A.; Arandiyan, H. The evaluation of autothermal methane reforming for hydrogen production over Ni/CeO2 catalysts. Int. J. Hydrog. Energy. 2018, 43, 22340–22346. [Google Scholar] [CrossRef]
- Shafaghat, H.; Tsang, Y.F.; Jeon, J.-K.; Kim, J.M.; Kim, Y.; Kim, S.; Park, Y.-K. In-situ hydrogenation of bio-oil/bio-oil phenolic compounds with secondary alcohols over a synthesized mesoporous Ni/CeO2 catalyst. Chem. Eng. J. 2020, 382, 122912. [Google Scholar] [CrossRef]
- Poolwong, J.; Del Gobbo, S.; D’Elia, V. Transesterification of dimethyl carbonate with glycerol by perovskite-based mixed metal oxide nanoparticles for the atom-efficient production of glycerol carbonate. J. Ind. Eng. Chem. 2021, 104, 43–60. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Shi, W.; Wei, C.; Song, X.; Yang, S.; Sun, Z. Baize-like CeO2 and NiO/CeO2 nanorod catalysts prepared by dealloying for CO oxidation. Nanotechnology 2016, 28, 045602. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Meng, Y.; Han, W.; Zhao, H.; Tang, Z. Morphology effects on surface chemical properties and lattice defects of Cu/CeO2 catalysts applied for low-temperature CO oxidation. Sci. Rep. 2019, 9, 12056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Miyazawa, T.; Nishikawa, J.; Kado, S.; Okumura, K.; Miyao, T.; Naito, S.; Kunimori, K.; Tomishige, K. Development of Ni catalysts for tar removal by steam gasification of biomass. Appl. Catal. B Environ. 2006, 68, 160–170. [Google Scholar] [CrossRef]
- Florea, I.; Feral-Martin, C.; Majimel, J.; Ihiawakrim, D.; Hirlimann, C.; Ersen, O. Three-Dimensional Tomographic Analyses of CeO2 Nanoparticles. Cryst. Growth Des. 2013, 13, 1110–1121. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Xu, G.; Zhang, Y.; Wang, C.; Lu, Q.; Ma, L. Highly efficient catalytic conversion of cellulose into acetol over Ni–Sn supported on nanosilica and the mechanism study. Green Chem. 2019, 21, 5647–5656. [Google Scholar] [CrossRef]
- Yan, N.; Wang, Y. Catalyst: Is the Amino Acid a New Frontier for Biorefineries? Chem 2019, 5, 739–741. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shang, N.; Gao, S.; Wang, J.; Meng, T.; Du, C.; Shen, T.; Huang, J.; Wu, Q.; Wang, H.; et al. Atomically Dispersed Co Catalyst for Efficient Hydrodeoxygenation of Lignin-Derived Species and Hydrogenation of Nitroaromatics. ACS Catal. 2020, 10, 8672–8682. [Google Scholar] [CrossRef]
- Dai, J.; Gözaydın, G.; Hu, C.; Yan, N. Catalytic Conversion of Chitosan to Glucosaminic Acid by Tandem Hydrolysis and Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 12399–12407. [Google Scholar] [CrossRef]
- Ferhan, M.; Tanguy, N.; Yan, N.; Sain, M. Comparison of Enzymatic, Alkaline, and UV/H2O2 Treatments for Extraction of Beetle-Infested Lodgepole Pine (BILP) and Aspen Bark Polyphenolic Extractives. ACS Sustain. Chem. Eng. 2014, 2, 165–172. [Google Scholar] [CrossRef]
- Lindfors, C.; Mäki-Arvela, P.; Paturi, P.; Aho, A.; Eränen, K.; Hemming, J.; Peurla, M.; Kubička, D.; Simakova, I.L.; Murzin, D.Y. Hydrodeoxygenation of Isoeugenol over Ni- and Co-Supported Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 14545–14560. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, D.; Singuru, R.; Venkataswamy, P.; Damma, D.; Reddy, B.M. Ceria Promoted Cu-Ni/SiO2 Catalyst for Selective Hydrodeoxygenation of Vanillin. ACS Omega. 2019, 4, 4770–4778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooms, R.; Dusselier, M.; Geboers, J.A.; Op de Beeck, B.; Verhaeven, R.; Gobechiya, E.; Martens, J.A.; Redl, A.; Sels, B.F. Conversion of sugars to ethylene glycol with nickel tungsten carbide in a fed-batch reactor: High productivity and reaction network elucidation. Green Chem. 2014, 16, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, B.A.; Stowers, C.C.; Pham, V.; Cox, B.M. The production of propionic acid, propanol and propylene via sugar fermentation: An industrial perspective on the progress, technical challenges and future outlook. Green Chem. 2014, 16, 1066–1076. [Google Scholar] [CrossRef]
- Liu, L.; Cao, J.; Nakagawa, Y.; Betchaku, M.; Tamura, M.; Yabushita, M.; Tomishige, K. Hydrodeoxygenation of C4–C6 sugar alcohols to diols or mono-alcohols with the retention of the carbon chain over a silica-supported tungsten oxide-modified platinum catalyst. Green Chem. 2021, 23, 5665–5679. [Google Scholar] [CrossRef]
- Yao, S.Y.; Xu, W.Q.; Johnston-Peck, A.C.; Zhao, F.Z.; Liu, Z.Y.; Luo, S.; Senanayake, S.D.; Martínez-Arias, A.; Liu, W.J.; Rodriguez, J.A. Morphological effects of the nanostructured ceria support on the activity and stability of CuO/CeO2 catalysts for the water-gas shift reaction. Phys. Chem. Chem. Phys. 2014, 16, 17183–17195. [Google Scholar] [CrossRef]
- Cao, T.; You, R.; Li, Z.; Zhang, X.; Li, D.; Chen, S.; Zhang, Z.; Huang, W. Morphology-dependent CeO2 catalysis in acetylene semihydrogenation reaction. Appl. Surf. Sci. 2020, 501, 144120. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, J.; Ma, K.; Zhang, Y.; Hu, Y.-M.; Kong, L.; Jia, A.-p.; Zhang, Z.; Huang, W.; Lu, J.-Q. Ceria morphology-dependent Pd-CeO2 interaction and catalysis in CO2 hydrogenation into formate. J. Catal. 2021, 397, 116–127. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Sun, J.; Oh, R.; Feng, J.; Shi, D.; Zhao, W.; Liu, S. Influence of CeO2 morphology on WO3/CeO2 catalyzed NO selective catalytic reduction by NH3. J. Energy Inst. 2020, 93, 1511–1518. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Wu, J.Y. Research on mechanism of deacetylation of chitin. Rare Metal Mat. Eng. 2008, 37, 718–722. [Google Scholar]
- Qi, M.; Chen, X.; Zhong, H.; Wu, J.; Jin, F. Base-Free, Vanadium-Catalyzed Conversion of Chitin into Acetic Acid under Low Oxygen Pressure. ACS Sustain. Chem. Eng. 2020, 8, 18661–18670. [Google Scholar] [CrossRef]
- Du, Y.; Zang, H.; Feng, Y.; Wang, K.; Lv, Y.; Liu, Z. Efficient catalytic system for converting N-acetyl-d-glucosamine into valuable chemical 3-acetylamino-5-acetylfuran. J. Mol. Liq. 2022, 347, 117970. [Google Scholar] [CrossRef]
- Szabolcs, Á.; Molnár, M.; Dibó, G.; Mika, L.T. Microwave-assisted conversion of carbohydrates to levulinic acid: An essential step in biomass conversion. Green Chem. 2013, 15, 439–445. [Google Scholar] [CrossRef]
- Hou, W.; Zhao, Q.; Liu, L. Selective conversion of chitin to levulinic acid catalyzed by ionic liquids: Distinctive effect of N-acetyl groups. Green Chem. 2020, 22, 62–70. [Google Scholar] [CrossRef]







| Materials | a SBET (m2/g) | b Ni (wt%) | c D (nm) | d Ƹ (%) | e L. P. (Å) | f Grain (Å) | g Crys. (%) | h,i [Ce3+]% | h,j [Ov] % | h,k [ID/ IF2g] |
|---|---|---|---|---|---|---|---|---|---|---|
| Ni/CeO2(R) | 50.5767 (136.7408) | 12.0 | 17.2 | 0.49 | 5.41 | 202 | 54.88 | 21.84 | 25.63 | 0.50 |
| Ni/CeO2(P) | 47.4038 (129.6053) | 11.8 | 10.8 | 0.77 | 5.41 | 127 | 54.96 | 30.60 | 35.81 | 0.97 |
| Ni/CeO2(C) | 28.4477 (51.6282) | 10.4 | 23.9 | 0.35 | 5.41 | 335 | 55.52 | 20.88 | 24.47 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Lu, L.; Chen, W.; Zheng, A.; Lei, A.; Dutta Chowdhury, A. Towards the Efficient Catalytic Valorization of Chitin to N-Acylethanolamine over Ni/CeO2 Catalyst: Exploring the Shape-Selective Reactivity. Catalysts 2022, 12, 460. https://doi.org/10.3390/catal12050460
Zheng Y, Lu L, Chen W, Zheng A, Lei A, Dutta Chowdhury A. Towards the Efficient Catalytic Valorization of Chitin to N-Acylethanolamine over Ni/CeO2 Catalyst: Exploring the Shape-Selective Reactivity. Catalysts. 2022; 12(5):460. https://doi.org/10.3390/catal12050460
Chicago/Turabian StyleZheng, Yifan, Lijun Lu, Wei Chen, Anmin Zheng, Aiwen Lei, and Abhishek Dutta Chowdhury. 2022. "Towards the Efficient Catalytic Valorization of Chitin to N-Acylethanolamine over Ni/CeO2 Catalyst: Exploring the Shape-Selective Reactivity" Catalysts 12, no. 5: 460. https://doi.org/10.3390/catal12050460
APA StyleZheng, Y., Lu, L., Chen, W., Zheng, A., Lei, A., & Dutta Chowdhury, A. (2022). Towards the Efficient Catalytic Valorization of Chitin to N-Acylethanolamine over Ni/CeO2 Catalyst: Exploring the Shape-Selective Reactivity. Catalysts, 12(5), 460. https://doi.org/10.3390/catal12050460