Copper Catalyzed Inverse Electron Demand [4+2] Cycloaddition for the Synthesis of Oxazines
Abstract
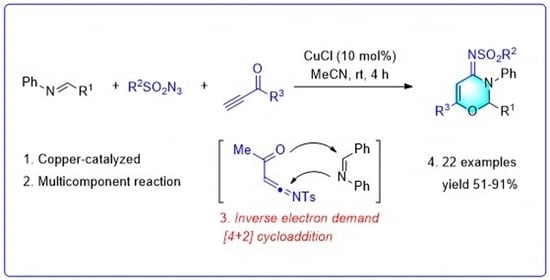
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of 2,3-Dihydro-4H-1,3-Oxazin-4-Ylidenes (4a–4v)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zinad, D.S.; Mahal, A.; Mohapatra, R.K.; Sarangi, A.K.; Pratama, M.R.F. Medicinal chemistry of oxazines as promising agents in drug discovery. Chem. Biol. Drug Des. 2020, 95, 16–47. [Google Scholar] [CrossRef] [PubMed]
- Li-Zhulanov, N.S.; Pavlova, A.V.; Korchagina, D.V.; Gatilov, Y.V.; Salakhutdinov, N.F. Synthesis of 1,3-Oxazine Derivatives Based on (–)-Isopulegol using the Ritter Reaction and Study of their Analgesic Activity. Chem. Heterocycl. Compd. 2020, 56, 936–941. [Google Scholar] [CrossRef]
- Abdel-Mageed, W.M.; Fayed, M.A.A.; Al-Saleem, M.S.M.; Al-Wahaibi, L.H.; Parvez, M.K.; Li, L.; Al-Dosaria, M.S.; Sayed, H.M. Novel polycyclic pyrroloquinazoline alkaloids from Anisotes trisulcus and their biological activity. J. Asian Nat. Prod. Res. 2020, 22, 1159–1167. [Google Scholar] [CrossRef]
- Popova, S.A.; Shevchenko, O.G.; Chukicheva, I.Y. Synthesis of new coumarin[1,3]oxazine derivatives of 7-hydroxy-6-isobornyl-4-methylcoumarin and their antioxidant activity. Chem. Biol. Drug Des. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-J.; Lu, J.-W.; Ho, L.-J.; Lai, J.-H.; Huang, H.-S.; Lee, C.-C.; Lin, T.-Y.; Lien, S.-B.; Lin, L.-C.; Chen, L.W.; et al. Anti-inflammatory and anti-osteoarthritis effects of Cm-02 and Ck-02. Biochem. Bioph. Res. Co. 2019, 517, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Fuchino, K.; Mitsuoka, Y.; Masui, M.; Kurose, N.; Yoshida, S.; Komano, K.; Yamamoto, T.; Ogawa, M.; Unemura, C.; Hosono, M. Rational Design of Novel 1,3-Oxazine Based β-Secretase (BACE1) Inhibitors: Incorporation of a Double Bond to Reduce P-gp Efflux Leading to Robust Aβ Reduction in the Brain. J. Med. Chem. 2018, 61, 5122–5137. [Google Scholar] [CrossRef] [PubMed]
- Gawalia, R.; Trivedib, J.; Bhansalic, S.; Bhosalea, R.; Sarkarc, D.; Mitrab, D. Design, synthesis, docking studies and biological screening of 2-thiazolyl substituted-2,3-dihydro-1H-naphtho[1,2-e][1,3]oxazines as potent HIV-1 reverse transcriptase inhibitors. Eur. J. Med. Chem. 2018, 157, 310–319. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Dawood, K.M.; Raslan, M.A. Heteroannulation Routes to Bioactive Pyrazolooxazines. Mini. Rev. Med. Chem. 2020, 24, 1943–1975. [Google Scholar]
- Wang, L.G.; Barth, C.W.; Kitts, C.H.; Mebrat, M.D.; Montaño, A.R.; House, B.J.; McCoy, M.E.; Antaris, A.L.; Galvis, S.N.; McDowall, I.; et al. Near-infrared nerve-binding fluorophores for buried nerve tissue imaging. Sci. Transl. Med. 2020, 12, 542. [Google Scholar] [CrossRef]
- Firpo, G.; Ramírez, M.L.; Faillace, M.S.; Brito, D.; Silva, E.; Costa, J.P.; Rodríguez, M.C.; Argüello, G.A.; Szakonyi, Z.; Fülöp, F.; et al. Evaluation of the Antioxidant Activity of Cis/Trans-N-Phenyl-1,4,4a,5,8,8a-Hexahydro-3,1-Benzoxazin-2-Imines. Antioxidants 2019, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Palchikov, V.A. Morpholines. Synthesis and biological activity. Russ. J. Org. Chem. 2013, 49, 787–814. [Google Scholar] [CrossRef]
- Shinde, P.V.; Kategaonkar, A.H.; Shingate, B.B.; Shingare, M.S. Polyethylene glycol (PEG) mediated expeditious synthetic route to 1,3-oxazine derivatives. Chin. Chem. Lett. 2011, 22, 915–918. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Zhang, R.-R.; Yin, W.-Z.; Yu, X.; Zhang, Y.-L.; Liu, P.; Gu, Y.-C.; Zhang, W.-H. Microwave-assisted Synthesis and antifungal activity of coumarin[8,7-e][1,3]oxazine derivatives. Mol. Divers. 2016, 20, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Botla, V.; Pilli, N.; Koude, D.; Misra, S.; Malapaka, C. Molecular Engineering of Tetracyclic 2,3-Dihydro-1H-benzo[2,3]-benzofuro[4,5-e][1,3]oxazine Derivatives: Evaluation for Potential Anticancer Agents. Arch. Pharm. Chem. 2017, 350, e1700169. [Google Scholar] [CrossRef] [PubMed]
- Vojacek, S.; Beese, K.; Alhalabi, Z.; Swyter, S.; Bodtke, A.; Schulzke, C.; Jung, M.; Sippl, W.; Link, A. Three-Component Aminoalkylations Yielding Dihydronaphthoxazine-Based Sirtuin Inhibitors: Scaffold Modification and Exploration of Space for Polar Side-Chains. Arch. Pharm. Chem. 2017, 350, e1700097. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-J.; Li, F.-H.; Wang, S.-Y.; Ji, S.-J. Palladium-Catalyzed Cascade Arene/Alkyne Annulation: Synthesis of Fluorene-Benzoxazine Derivatives. Org. Lett. 2016, 18, 4810–4813. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Gridnev, I.D.; Terada, M.; Nakamura, I. Au-Catalyzed Skeletal Rearrangement of O-Propargylic Oximes via N-O Bond Cleavage with the Aid of a Brønsted Base Cocatalyst. Chem. Sci. 2019, 10, 5283–5289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-Y.; Han, J.-B.; Wang, L.; Tang, X.-Y. Lewis Acid Catalyzed [4 + 2] Cycloaddition of N-Tosylhydrazones with ortho-Quinone Methides. J. Org. Chem. 2019, 84, 14258–14269. [Google Scholar] [CrossRef]
- Palacios, F.; Herra, E.; Rubiales, G.; Ezpeleta, J.M. Cycloaddition Reaction of 2-Azadienes Derived from â-Amino Acids with Electron-Rich and Electron-Deficient Alkenes and Carbonyl Compounds. Synthesis of Pyridine and 1,3-Oxazine Derivatives. J. Org. Chem. 2002, 67, 2131–2135. [Google Scholar] [CrossRef]
- Kikuchi, J.; Ye, H.; Terada, M. Chiral Phosphoric Acid Catalyzed Enantioselective [4 + 2] Cycloaddition Reaction of α-Fluorostyrenes with Imines. Org. Lett. 2020, 22, 8957–8961. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Y.; Bu, Q.; Li, L.; Zhou, B.; Huang, Z. Tandem CuAAC/ring cleavage/[4 + 2] annulation reaction to synthesize dihydrooxazines and conversion to 2-aminopyrimidines. Org. Lett. 2022, 24, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, R. Recent Developments in Catalytic Asymmetric Inverse-Electron-Demand Diels–Alder Reaction. Chem. Rev. 2013, 113, 5515–5546. [Google Scholar] [CrossRef]
- Guney, T.; Lee, J.J.; Kraus, G.A. First Inverse Electron-Demand Diels−Alder Methodology of 3-Chloroindoles and Methyl Coumalate to Carbazoles. Org. Lett. 2014, 16, 1124–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, S.; Krause, J.; Mandal, T.; Zhao, C.-G. Inverse-Electron-Demand Hetero-Diels-Alder Reaction of β,γ-Unsaturated α-Ketophosphonates Catalyzed by Prolinal Dithioacetals. Org. Lett. 2007, 9, 2745–2748. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Yang, S.; Meng, M.; Zhan, X.; Zeng, M.; Fang, X. Bifunctional Thiourea-Catalyzed Asymmetric Inverse-Electron-Demand Diels−Alder Reaction of Allyl Ketones and Vinyl 1,2-Diketones via Dienolate Intermediate. Org. Lett. 2019, 21, 1979–1983. [Google Scholar] [CrossRef]
- Choi, Y.; Ishikawa, H.; Velcicky, J.; Elliott, G.I.; Miller, M.M.; Boger, D.L. Total Synthesis of (−)- and ent-(+)-Vindoline. Org. Lett. 2005, 7, 4539–4542. [Google Scholar] [CrossRef] [PubMed]
- Saktura, M.; Grzelak, P.; Dybowska, J.; Albrecht, Ł. Asymmetric Synthesis of [2.2.2]-Bicyclic Lactones via All-Carbon Inverse-Electron-Demand Diels−Alder Reaction. Org. Lett. 2020, 22, 1813–1817. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Liu, C.; Zhou, J.; Zhan, R.; Chen, W.; Huang, H. Asymmetric Inverse-Electron-Demand Diels-Alder Reaction of β,γ-Unsaturated Amides through Dienolate Catalysis. Org. Lett. 2019, 21, 7337–7341. [Google Scholar] [CrossRef]
- Gunawardene, P.N.; Luo, W.; Polgar, A.M.; Corrigan, J.F.; Workentin, M.S. Highly Electron-Deficient Pyridinium-Nitrones for Rapid and Tunable Inverse-Electron-Demand Strain-Promoted Alkyne-Nitrone Cycloaddition. Org. Lett. 2019, 21, 5547–5551. [Google Scholar]
- Zhao, J.-J.; Sun, S.-B.; He, S.-H.; Wu, Q.; Shi, F. Catalytic Asymmetric Inverse-Electron-Demand Oxa-Diels-Alder Reaction of In Situ Generated ortho-Quinone Methides with 3-Methyl-2-Vinylindoles. Angew. Chem. Int. Ed. 2015, 54, 5460–5464. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Zhang, H.; Gao, Y.; Mo, L.; Wang, S.; Yao, Z.-J. Asymmetric Cascade Annulation Based on Enantioselective Oxa-Diels-Alder Cycloaddition of in Situ Generated Isochromenyliums by Cooperative Binary Catalysis of Pd(OAc)2 and (S)-Trip. J. Am. Chem. Soc. 2013, 135, 11402–11407. [Google Scholar] [CrossRef] [PubMed]
- Gademann, K.; Chavez, D.E.; Jacobsen, E.N. Highly Enantioselective Inverse-Electron-Demand Hetero-Diels-Alder Reactions of α,β-Unsaturated Aldehydes. Angew. Chem. Int. Ed. 2002, 41, 3059–3061. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, L.; Yin, C.; Wang, Z.; Liu, X.; Feng, X. The N,N′-dioxide/Ni(ii)-catalyzed asymmetric inverse-electron-demand hetero-Diels-Alder reaction of methyleneindolinones with hetero-substituted alkenes. Chem. Commun. 2015, 51, 11689–11692. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.-B.; Qi, Y.; Jing, Z.-R.; Wang, S.-H.; Tu, Y.-Q.; Zhu, D.-Y.; Zhang, F.-M. Efficient Oxa-Diels-Alder/Semipinacol Rearrangement/Aldol Cascade Reaction: Short Approach to Polycyclic Architectures. Org. Lett. 2015, 17, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Suzuki, T.; Sakakura, A.; Ishihara, K. Catalytic Enantioselective Inverse Electron Demand Hetero-Diels-Alder Reaction with Allylsilanes. Angew. Chem. Int. Ed. 2014, 53, 6131–6134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, L.; Zhang, P.; Zhong, Y.; Wang, R. Catalytic Asymmetric β,γ Activation of α,β-Unsaturated γ-Butyrolactams: Direct Approach to β,γ-Functionalized Dihydropyranopyrrolidin-2-ones. Angew. Chem. Int. Ed. 2013, 52, 11329–11333. [Google Scholar] [CrossRef] [PubMed]
- Nájera, C.; Sydnes, L.K.; Yus, M. Conjugated Ynones in Organic Synthesis. Chem. Rev. 2019, 119, 11110–11244. [Google Scholar] [CrossRef]
- Nallagangula, M.; Namitharan, K. Copper-Catalyzed Sulfonyl Azide-Alkyne Cycloaddition Reactions: Simultaneous Generation and Trapping of Copper-Triazoles and -Ketenimines for the Synthesis of Triazolopyrimidines. Org. Lett. 2017, 19, 3536–3539. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Rudd, M.T.; Reddy, M.V.R. Stereoselective synthesis of hex-2-(E)-en-4-yn-1,6-dioates and E,Z-muconic acid diesters via organo-catalyzed self-coupling of propiolates. Tetrahedron Lett. 2005, 46, 2547–2549. [Google Scholar] [CrossRef]
- Samaraj, E.; Balaraman, E.; Manickam, S. Functional POM-catalyst for selective oxidative dehydrogenative couplings under aerobic conditions. Mol. Catal. 2021, 502, 111396–111405. [Google Scholar] [CrossRef]
- Das, D.; Samanta, R. Iridium(III)-Catalyzed Regiocontrolled Direct Amidation of Isoquinolones and Pyridones. Adv. Synth. Catal. 2018, 360, 379–384. [Google Scholar] [CrossRef]
- Chernyak, D.; Gadamsetty, S.B.; Gevorgyan, V. Low Temperature Organocopper-Mediated Two-Component Cross Coupling/Cycloisomerization Approach Toward N-Fused Heterocycles. Org. Lett. 2008, 10, 2307–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]





 | ||||||
|---|---|---|---|---|---|---|
| Entry | Cat. (10 mol%) | Base (0.75 mmol) | Solvent (2 mL) | Temp. (°C) | Time (h) | Yield (%) b |
| 1 | CuCI | - | Acetone | rt | 4.0 | 21 |
| 2 | CuCI | - | THF | rt | 4.0 | 65 |
| 3 | CuCI | - | DMF | rt | 4.0 | 12 |
| 4 | CuCI | - | DCM | rt | 4.0 | 21 |
| 5 | CuCI | - | DMSO | rt | 4.0 | 15 |
| 6 | CuCI | - | MeCN | rt | 4.0 | 84 |
| 7 | CuI | - | MeCN | rt | 4.0 | 78 |
| 8 | Cu(OAc)2 | - | MeCN | rt | 4.0 | 71 |
| 9 | Cu(acac)2 | - | MeCN | rt | 4.0 | 22 |
| 10 | CuO | - | MeCN | rt | 4.0 | Trace |
| 11 | CuBr | - | MeCN | rt | 4.0 | 65 |
| 12 | Cu(SO4)2 | - | MeCN | rt | 4.0 | Trace |
| 13 | Cu(TFA)2 | - | MeCN | rt | 4.0 | 32 |
| 14 | AgTFA | - | MeCN | rt | 4.0 | 0 |
| 15 | CuCI | DMAP | MeCN | rt | 4.0 | Trace |
| 16 | CuCI | TsOH | MeCN | rt | 4.0 | Trace |
| 17 | CuCI | K2CO3 | MeCN | rt | 4.0 | 12 |
| 18 | CuCI | HOAc | MeCN | rt | 4.0 | 24 |
| 19 | CuCI | Et3N | MeCN | rt | 4.0 | 42 |
| 20 | CuCI | - | MeCN | 40 | 4.0 | 76 |
| 21 | CuCI | - | MeCN | 60 | 4.0 | 62 |
| 22 | CuCI | - | MeCN | 80 | 4.0 | 41 |
| 23 | CuCI | - | MeCN | rt | 3.0 | 77 |
| 24 | CuCI | - | MeCN | rt | 5.0 | 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Zhou, Z.; Zhao, Y.; Luo, D.; Luo, X.; Luo, H.; Cui, L.; Li, L. Copper Catalyzed Inverse Electron Demand [4+2] Cycloaddition for the Synthesis of Oxazines. Catalysts 2022, 12, 526. https://doi.org/10.3390/catal12050526
Yang W, Zhou Z, Zhao Y, Luo D, Luo X, Luo H, Cui L, Li L. Copper Catalyzed Inverse Electron Demand [4+2] Cycloaddition for the Synthesis of Oxazines. Catalysts. 2022; 12(5):526. https://doi.org/10.3390/catal12050526
Chicago/Turabian StyleYang, Weiguang, Zitong Zhou, Yu Zhao, Danyang Luo, Xiai Luo, Hui Luo, Liao Cui, and Li Li. 2022. "Copper Catalyzed Inverse Electron Demand [4+2] Cycloaddition for the Synthesis of Oxazines" Catalysts 12, no. 5: 526. https://doi.org/10.3390/catal12050526
APA StyleYang, W., Zhou, Z., Zhao, Y., Luo, D., Luo, X., Luo, H., Cui, L., & Li, L. (2022). Copper Catalyzed Inverse Electron Demand [4+2] Cycloaddition for the Synthesis of Oxazines. Catalysts, 12(5), 526. https://doi.org/10.3390/catal12050526