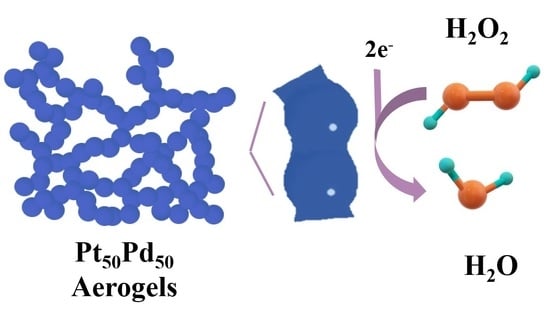
Pt–Pd Bimetallic Aerogel as High-Performance Electrocatalyst for Nonenzymatic Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of PtxPdy Bimetallic Aerogels and Pt, Pd Monometallic Aerogels
2.2. Electrocatalytic Reduction of H2O2 by the Metal Aerogel Catalyst Modified Glassy Carbon Electrodes
2.3. Effect of pH in H2O2 Detection by the Pt50Pd50 Aerogel Catalyst Modified Glassy Carbon Electrode
2.4. Amperometric Sensing of H2O2 by PtxPdy Bimetallic Aerogels and Pt, Pd Monometallic Aerogels
2.5. Selectivity, Reproducibility, Repeatability, and Stability of the As-Prepared Sensors
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Synthesis of PtxPdy Bimetallic Aerogels and Pt, Pd Monometallic Aerogels
3.3. Apparatus
3.4. Electrochemical Performance Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, L.; Xu, W.; Yan, H.; Wu, Y.; Liu, C.; Du, D.; Lin, Y.; Zhu, C. Fe–N–C single-atom nanozymes for the intracellular hydrogen peroxide detection. Anal. Chem. 2019, 91, 11994–11999. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, Y.; Zhang, C.; Zhao, Y.; Lu, C.; Yin, B.; Yang, Y.; Gong, X.; Teng, L.; Liu, Y.; et al. An acidity-unlocked magnetic nanoplatform enables self-boosting ROS generation through upregulation of lactate for imaging-guided highly specific chemodynamic therapy. Angew. Chem. Int. Ed. 2021, 60, 9562–9572. [Google Scholar] [CrossRef] [PubMed]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; Ponce de León, C.; Walsh, F.C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P.; Prato, M. The rise of hydrogen peroxide as the main product by metal-free catalysis in oxygen reductions. Adv. Mater. 2019, 31, 1802920. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.-I. Ros-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Zhang, C.; Gao, X.; Zhuang, Z.; Du, C.; Chen, W. 3D network and 2D paper of reduced graphene oxide/Cu2O composite for electrochemical sensing of hydrogen peroxide. Anal. Chem. 2018, 90, 1983–1991. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.; Hofferber, E.M.; Stapleton, J.A.; Iverson, N.M. Hydrogen peroxide sensors for biomedical applications. Chemosensors 2019, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Tantawi, O.; Baalbaki, A.; El Asmar, R.; Ghauch, A. A rapid and economical method for the quantification of hydrogen peroxide (H2O2) using a modified HPLC apparatus. Sci. Total Environ. 2019, 654, 107–117. [Google Scholar] [CrossRef]
- Trujillo, R.M.; Barraza, D.E.; Zamora, M.L.; Cattani-Scholz, A.; Madrid, R.E. Nanostructures in hydrogen peroxide sensing. Sensors 2021, 21, 2204. [Google Scholar] [CrossRef]
- Han, T.; Zhang, Y.; Xu, J.; Dong, J.; Liu, C.-C. Monodisperse AuM (M = Pd, Rh, Pt) bimetallic nanocrystals for enhanced electrochemical detection of H2O2. Sens. Actuators B 2015, 207, 404–412. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, M.; Meng, X.; Xiang, J.; Wang, L.; Ren, Q.; Guo, S. Graphene/intermetallic PtPb nanoplates composites for boosting electrochemical detection of H2O2 released from cells. Anal. Chem. 2017, 89, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Liu, H.; Sun, B.; Xiao, B.; Jiang, Y.; Jin, C.; Yuan, A.; Zhang, H.; Yang, D. Ion-templated fabrication of Pt-Cu alloy octahedra with controlled compositions for electrochemical detection of H2O2. J. Alloys Compd. 2019, 788, 1334–1340. [Google Scholar] [CrossRef]
- Liu, W.; Hiekel, K.; Hübner, R.; Sun, H.; Ferancova, A.; Sillanpää, M. Pt and Au bimetallic and monometallic nanostructured amperometric sensors for direct detection of hydrogen peroxide: Influences of bimetallic effect and silica support. Sens. Actuators B 2018, 255, 1325–1334. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Gong, S.; Chen, Y.; Xie, R.; Wu, Q.; Tao, J.; Meng, F.; Zhao, P. A novel non-enzymatic electrochemical biosensor based on the nanohybrid of bimetallic PdCu nanoparticles/carbon black for highly sensitive detection of H2O2 released from living cells. Sens. Actuators B 2019, 290, 249–257. [Google Scholar] [CrossRef]
- Wu, L.; Yin, W.; Tan, X.; Wang, P.; Ding, F.; Zhang, H.; Wang, B.; Zhang, W.; Han, H. Direct reduction of HAuCl4 for the visual detection of intracellular hydrogen peroxide based on Au-Pt/SiO2 nanospheres. Sens. Actuators B 2017, 248, 367–373. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, M.; Qin, Y.; Zhu, S.; Li, Y.; Xu, N.; Meng, X.; Ren, Q.; Wang, L.; Guo, S. Atomic-thick PtNi nanowires assembled on graphene for high-sensitivity extracellular hydrogen peroxide sensors. ACS Appl. Mater. Interfaces 2017, 9, 34715–34721. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Dong, W.; Ren, Y.; Zhang, C.; Chen, Q. Preparation of nano Au and Pt alloy microspheres decorated with reduced graphene oxide for nonenzymatic hydrogen peroxide sensing. Langmuir 2018, 34, 2235–2244. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Gesser, H.D.; Goswami, P.C. Aerogels and related porous materials. Chem. Rev. 1989, 89, 765–788. [Google Scholar] [CrossRef]
- Cai, B.; Dianat, A.; Hubner, R.; Liu, W.; Wen, D.; Benad, A.; Sonntag, L.; Gemming, T.; Cuniberti, G.; Eychmuller, A. Multimetallic hierarchical aerogels: Shape engineering of the building blocks for efficient electrocatalysis. Adv. Mater. 2017, 29, 1605254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Herrmann, A.K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmuller, A. Noble metal aerogels-synthesis, characterization, and application as electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, R.; Hübner, R.; Hu, Y.; Eychmüller, A. A roadmap for 3D metal aerogels: Materials design and application attempts. Matter 2021, 4, 54–94. [Google Scholar] [CrossRef]
- Du, R.; Fan, X.; Jin, X.; Hübner, R.; Hu, Y.; Eychmüller, A. Emerging noble metal aerogels: State of the art and a look forward. Matter 2019, 1, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.; Eychmuller, A. Promoting electrocatalysis upon aerogels. Adv. Mater. 2019, 31, 1804881. [Google Scholar] [CrossRef] [Green Version]
- Dubale, A.A.; Zheng, Y.; Wang, H.; Hubner, R.; Li, Y.; Yang, J.; Zhang, J.; Sethi, N.K.; He, L.; Zheng, Z.; et al. High-performance bismuth-doped nickel aerogel electrocatalyst for the methanol oxidation reaction. Angew. Chem. Int. Ed. 2020, 59, 13891–13899. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, J.; Lu, X.; Wang, H.; Dubale, A.A.; Li, Y.; Jin, Z.; Lou, D.; Sethi, N.K.; Ye, Y.; et al. Boosting both electrocatalytic activity and durability of metal aerogels via intrinsic hierarchical porosity and continuous conductive network backbone preservation. Adv. Energy Mater. 2020, 11, 2002276. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.K.; Geiger, D.; Borchardt, L.; Simon, F.; Kaskel, S.; Gaponik, N.; Eychmuller, A. High-performance electrocatalysis on palladium aerogels. Angew. Chem. Int. Ed. 2012, 51, 5743–5747. [Google Scholar] [CrossRef]
- Du, R.; Jin, W.; Wu, H.; Hübner, R.; Zhou, L.; Xue, G.; Hu, Y.; Eychmüller, A. Rapid synthesis of gold–palladium core–shell aerogels for selective and robust electrochemical CO2 reduction. J. Mater. Chem. A Chem. 2021, 9, 17189–17197. [Google Scholar] [CrossRef]
- Liu, W.; Rodriguez, P.; Borchardt, L.; Foelske, A.; Yuan, J.; Herrmann, A.K.; Geiger, D.; Zheng, Z.; Kaskel, S.; Gaponik, N.; et al. Bimetallic aerogels: High-performance electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 52, 9849–9852. [Google Scholar] [CrossRef]
- Liu, W.; Haubold, D.; Rutkowski, B.; Oschatz, M.; Hübner, R.; Werheid, M.; Ziegler, C.; Sonntag, L.; Liu, S.; Zheng, Z.; et al. Self-supporting hierarchical porous PtAg alloy nanotubular aerogels as highly active and durable electrocatalysts. Chem. Mater. 2016, 28, 6477–6483. [Google Scholar] [CrossRef]
- Wen, D.; Liu, W.; Haubold, D.; Zhu, C.; Oschatz, M.; Holzschuh, M.; Wolf, A.; Simon, F.; Kaskel, S.; Eychmuller, A. Gold aerogels: Three-dimensional assembly of nanoparticles and their use as electrocatalytic interfaces. ACS Nano 2016, 10, 2559–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Shao, Z.-G.; Lu, W.; Xie, F.; Qin, X.; Yi, B. Electrochemical preparation and characterization of PdPt nanocages with improved electrocatalytic activity toward oxygen reduction reaction. Electrochim. Acta 2013, 103, 66–76. [Google Scholar] [CrossRef]
- Luo, M.; Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Chem. 2017, 2, 17095. [Google Scholar] [CrossRef]
- Veisz, B.; Tóth, L.; Teschner, D.; Paál, Z.; Győrffy, N.; Wild, U.; Schlögl, R. Palladium-platinum powder catalysts manufactured by colloid synthesis. J. Mol. Catal. A Chem. 2005, 238, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Ruban, A.; Hammer, B.; Stoltze, P.; Skriver, H.L.; Nørskov, J.K. Surface electronic structure and reactivity of transition and noble metals1communication presented at the first francqui colloquium. J. Mol. Catal. A Chem. 1997, 115, 421–429. [Google Scholar] [CrossRef]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, J.; Yang, S.; Liu, T.; Tao, K.; Chang, H. A silver wire aerogel promotes hydrogen peroxide reduction for fuel cells and electrochemical sensors. J. Mater. Chem. A Chem. 2019, 7, 11497–11505. [Google Scholar] [CrossRef]
- Sha, R.; Vishnu, N.; Badhulika, S. Bimetallic Pt-Pd nanostructures supported on MoS2 as an ultra-high performance electrocatalyst for methanol oxidation and nonenzymatic determination of hydrogen peroxide. Microchim. Acta. 2018, 185, 399. [Google Scholar] [CrossRef]
- Chen, K.J.; Chandrasekara Pillai, K.; Rick, J.; Pan, C.J.; Wang, S.H.; Liu, C.C.; Hwang, B.J. Bimetallic PtM (M=Pd, Ir) nanoparticle decorated multi-walled carbon nanotube enzyme-free, mediator-less amperometric sensor for H2O2. Biosens. Bioelectron. 2012, 33, 120–127. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, D.; Li, C.; Zou, L.; Ye, B. Graphene blended with SnO2 and Pd-Pt nanocages for sensitive non-enzymatic electrochemical detection of H2O2 released from living cells. Anal. Chim. Acta. 2018, 1014, 10–18. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; He, H.; Shi, L.; Cai, X.; Lan, M. Pt-Pd bimetallic nanocoral modified carbon fiber microelectrode as a sensitive hydrogen peroxide sensor for cellular detection. Sens. Actuators B 2018, 260, 174–182. [Google Scholar] [CrossRef]
- Qi, H.; Song, J.; Fu, Y.; Wu, X.; Qi, H. Highly dispersive Pt-Pd nanoparticles on graphene oxide sheathed carbon fiber microelectrodes for electrochemical detection of H2O2 released from living cells. Nanotechnology 2020, 31, 135503. [Google Scholar] [CrossRef]
- Sun, X.; Guo, S.; Liu, Y.; Sun, S. Dumbbell-like PtPd -Fe3O4 nanoparticles for enhanced electrochemical detection of H2O2. Nano Lett. 2012, 12, 4859–4863. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Y.; Wu, S.; Cai, Y.; Liu, H.; Zhang, J.; Yang, C.; He, G.; Xiao, W.; Li, L.; et al. One-pot synthesis of cubic PtPdCu nanocages with enhanced electrocatalytic activity for reduction of H2O2. RSC Adv. 2017, 7, 34071–34076. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Zuo, J.; Tan, L.; Pang, H.; Ma, H. Enzymeless electrochemical determination of hydrogen peroxide at a heteropolyanion-based composite film electrode. New J. Chem. 2019, 43, 1053–1062. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Zhang, D.; Ma, M.; Wang, W.; Chen, Q. Nano-assemblies consisting of Pd/Pt nanodendrites and poly (diallyldimethylammonium chloride)-coated reduced graphene oxide on glassy carbon electrode for hydrogen peroxide sensors. Mater. Sci. Eng. C 2016, 58, 1246–1254. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, H.; Wang, C.; Yang, M.; Zhou, A.; Duan, H. Ultrasonic-electrodeposition of PtPd alloy nanoparticles on ionic liquid-functionalized graphene paper: Towards a flexible and versatile nanohybrid electrode. Nanoscale 2016, 8, 1523–1534. [Google Scholar] [CrossRef]









| Aerogels | Expected Pt/Pd (at%) | EDS Pt/Pd (at%) | XPS (at%) | D-Band Center Location (eV) | ||
|---|---|---|---|---|---|---|
| Pt/Pd | Pt/PtO | Pd/PdO | ||||
| Pd | 0/100 | - | - | - | 57.3/42.7 | 2.47 |
| Pt20Pd80 | 20/80 | 20.4/79.7 | 24.9/75.1 | 80.2/19.8 | 62.0/38.0 | 2.74 |
| Pt50Pd50 | 50/50 | 50.6/49.4 | 59.6/40.4 | 75.2/24.8 | 83.7/16.3 | 3.02 |
| Pt80Pd20 | 80/20 | 83.2/16.8 | 85.7/14.3 | 70.2/29.8 | 64.3/35.7 | 3.11 |
| Pt | 100/0 | - | - | 83.3/16.7 | - | 3.27 |
| Electrode | CV Epc (V) | Eapplied (V vs. Ag/AgCl) | Linear Range (μM) | LOD (μM) | Sensitivity (mA mM−1 cm−2) | tresponse (s) | Substrate Materials | Ref |
|---|---|---|---|---|---|---|---|---|
| Pt50Pd50 aerogel | −0.023 | −0.05 | 5.1–3190 | 2.21 | 0.19 | 3 | - | This work |
| Pt80Pd20 aerogel | −0.115 | −0.05 | 5.1–4200 | 2.73 | 0.16 | 2 | - | This work |
| Pt20Pd80 aerogel | −0.014 | −0.05 | 10–3700 | 6.73 | 0.114 | 3 | - | This work |
| Pt aerogel | −0.136 | −0.05 | 5.2–2700 | 4.21 | 0.143 | ≤5 s | - | This work |
| Pd aerogel | 0.004 | −0.05 | 19–5576 | 21.3 | 0.047 | ≤5 s | - | This work |
| Ag wire aerogel | ~−0.14 | −0.46 | 0–800 | 2.1 | 0.42 | ~50 s | - | [38] |
| Pt–Pd/CFME | not obvious | −0.4 | 5–3920 | 0.48 | 10.4 | ≤5 s | carbon fiber | [42] |
| Pt–Pd NPs/GO-CFMs | ~−0.2 | −0.2 | 1–35 | 0.3 | 0.98 μA mM−1 | - | graphene oxide sheathed carbon fiber | [43] |
| Pt–Pb/graphene | ~−0.19 | −0.2 | 0.002–2516 | 0.002 | 1.82, 0.91, 4.05 | 2 | graphene | [12] |
| Pt (750 s) Pd/MoS2 | not obvious | −0.35 | 10–80 | 3.4 | 7.64 | - | MoS2 | [39] |
| Pt48Pd52−Fe3O4 NPs | −0.456 | −0.25 | 0.02–0.1 0.1–2.0 2.0–14,000 | 0.005 | - | 2 | Fe3O4 | [44] |
| PdPt NCs@SGN | ~0.13 | −0.06 | 1–300 | 0.3 | 14,968.75 | 3 | SnO2/graphene nanosheets | [41] |
| Pt–PdCu NCs | ~0.05 | 0.05 | 1.5–1160 | 1.5 | 0.562 | - | none | [45] |
| [PB/WV-Pt@Pd]6 | <−0.5 | −0.3 | 0.4–2650 | 0.1 | 0.0551 | PW9V3O403− | [46] | |
| Pd core–Pt NDs-rGO | ~0.02 | 0.018 | 5–500 | 0.027 | 0.672 | ≤5 s | PDDA–rGO | [47] |
| Pt–Pd/IL-rGOP(II) | −0.35 | 0.2 * | 0.1–37.6 | 0.01 | ≤5 s | IL–graphene paper | [48] * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Zheng, Y.; Yang, J.; Lou, D.; Li, J.; Sun, Y.; Liu, W. Pt–Pd Bimetallic Aerogel as High-Performance Electrocatalyst for Nonenzymatic Detection of Hydrogen Peroxide. Catalysts 2022, 12, 528. https://doi.org/10.3390/catal12050528
Pan C, Zheng Y, Yang J, Lou D, Li J, Sun Y, Liu W. Pt–Pd Bimetallic Aerogel as High-Performance Electrocatalyst for Nonenzymatic Detection of Hydrogen Peroxide. Catalysts. 2022; 12(5):528. https://doi.org/10.3390/catal12050528
Chicago/Turabian StylePan, Chuxuan, Yuanyuan Zheng, Jing Yang, Dongyang Lou, Jian Li, Yujing Sun, and Wei Liu. 2022. "Pt–Pd Bimetallic Aerogel as High-Performance Electrocatalyst for Nonenzymatic Detection of Hydrogen Peroxide" Catalysts 12, no. 5: 528. https://doi.org/10.3390/catal12050528
APA StylePan, C., Zheng, Y., Yang, J., Lou, D., Li, J., Sun, Y., & Liu, W. (2022). Pt–Pd Bimetallic Aerogel as High-Performance Electrocatalyst for Nonenzymatic Detection of Hydrogen Peroxide. Catalysts, 12(5), 528. https://doi.org/10.3390/catal12050528