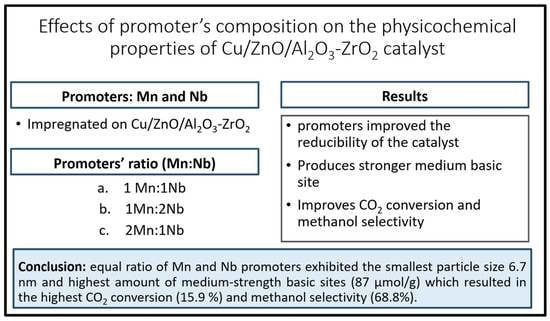
Effects of Promoter’s Composition on the Physicochemical Properties of Cu/ZnO/Al2O3-ZrO2 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Evaluation
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NASA. Global Climate Change. Carbon Dioxide Concentration. 2019. Available online: https://climate.nasa.gov/vital-signs/carbon-dioxide/ (accessed on 13 December 2020).
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Oh, S.-Y.; Park, M.-J.; Lee, W.B. Direct utilization of CO2 via methanol synthesis for natural gas fields with high CO2 concentration. J. Nat. Gas Sci. Eng. 2021, 96, 104308. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C.-J. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2020, 44, 101413. [Google Scholar] [CrossRef]
- Goeppert, A.; Olah, G.A.; Surya Prakash, G.K. Toward, a Sustainable Carbon Cycle: The Methanol Economy. In Green Chemistry: An Inclusive Approach; Török, B., Dransfield, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 919–962. [Google Scholar]
- Biswal, T.; Shadangi, K.P.; Sarangi, P.K.; Srivastava, R.K. Conversion of carbon dioxide to methanol: A comprehensive review. Chemosphere 2022, 298, 134299. [Google Scholar] [CrossRef]
- Niu, J.; Liu, H.; Jin, Y.; Fan, B.; Qi, W.; Ran, J. Comprehensive review of Cu-based CO2 hydrogenation to CH3OH: Insights from experimental work and theoretical analysis. Int. J. Hydrogen Energy 2022, 47, 9183–9200. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Garcia, R.; Fierro, J.L.G.; Navarro, R.M. Structure and activity of Cu/ZnO catalysts co-modified with aluminium and gallium for methanol synthesis. Catal. Today 2020, 355, 870–881. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, R.; Sharma, T.; Nguyen, D.D.; Cheng, C.K.; Lam, S.S.; Xia, C.; Nadda, A.K. Integrated catalytic insights into methanol production: Sustainable framework for CO2 conversion. J. Environ. Manag. 2021, 289, 112468. [Google Scholar] [CrossRef]
- Alsuhaibani, A.S.; Afzal, S.; Challiwala, M.; Elbashir, N.O.; El-Halwagi, M.M. The impact of the development of catalyst and reaction system of the methanol synthesis stage on the overall profitability of the entire plant: A techno-economic study. Catal. Today 2020, 343, 191–198. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Ramirez, A.; Navajas, A.; Gevers, L.; Brunialti, S.; Gandía, L.M.; Aguayo, A.T.; Sarathy, S.M.; Gascon, J. A techno-economic and life cycle assessment for the production of green methanol from CO2: Catalyst and process bottlenecks. J. Energy Chem. 2022, 68, 255–266. [Google Scholar] [CrossRef]
- Rahmatmand, B.; Rahimpour, M.R.; Keshavarz, P. Introducing a novel process to enhance the syngas conversion to methanol over Cu/ZnO/Al2O3 catalyst. Fuel Process. Technol. 2019, 193, 159–179. [Google Scholar] [CrossRef]
- Bowker, M.; Hadden, R.A.; Houghton, H.; Hyland, J.N.K.; Waugh, K.C. The Mechanism of Methanol Synthesis on Copper/Zinc Oxide/Alumina Catalysts. J. Catal. 1998, 109, 263–273. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Xu, X.; Yang, P.; Miao, P.; Zhang, Y.; Sun, Q. Effect of Al-containing precursors on Cu/ZnO/Al2O3 catalyst for methanol production. Fuel Process. Technol. 2018, 178, 148–155. [Google Scholar] [CrossRef]
- Choi, Y.; Futagami, K.; Fujitani, T.; Nakamura, J. The difference in the active sites for CO2 and CO hydrogenations on Cu/ZnO-based methanol synthesis catalysts. Catal. Lett. 2001, 73, 27–31. [Google Scholar] [CrossRef]
- Dasireddy, V.D.; Likozar, B. The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity. Renew. Energy 2019, 140, 452–460. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Wang, H.; Sun, Y. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. J. Catal. 2013, 298, 51–60. [Google Scholar] [CrossRef]
- Ren, H.; Xu, C.; Zhao, H.; Wang, Y.; Liu, J.; Liu, J. Methanol synthesis from CO2 hydrogenation over Cu/g-Al2O3 catalysts modified by ZnO, ZrO2 and MgO. J. Ind. Eng. Chem. 2015, 28, 261–267. [Google Scholar] [CrossRef]
- Guo, T.; Guo, Q.; Li, S.; Hu, Y.; Yun, S.; Qian, Y. Effect of surface basicity over the supported Cu-ZnO catalysts on hydrogenation of CO2 to methanol. J. Catal. 2022, 407, 312–321. [Google Scholar] [CrossRef]
- Marcosa, F.C.F.; Lin, L.; Betancourt, L.E.; Senanayake, S.D.; Rodriguez, J.A.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Insights into the methanol synthesis mechanism via CO2 hydrogenation over Cu-ZnO-ZrO2 catalysts: Effects of surfactant/Cu-Zn-Zr molar ratio. J. CO2 Util. 2020, 41, 1012015. [Google Scholar] [CrossRef]
- Xaba, B.; Mahomed, A.; Friedrich, H. The effect of CO2 and H2 adsorption strength and capacity on the performance of Ga and Zr modified Cu-Zn catalysts for CO2 hydrogenation to methanol. J. Environ. Chem. Eng. 2021, 9, 104834. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2013, 468, 442–452. [Google Scholar] [CrossRef]
- Li, C.; Yuan, X.; Fujimoto, K. Development of highly stable catalyst for methanol synthesis from carbon dioxide. Appl. Catal. A Gen. 2014, 469, 306–311. [Google Scholar] [CrossRef]
- Słoczyn´ski, J.; Grabowski, R.; Olszewski, P.; Kozłowska, A.; Stoch, J.; Lachowska, M.; Skrzypek, J. Effect of metal oxide additives on the activity and stability of Cu/ZnO/ZrO2 catalysts in the synthesis of methanol from CO2 and H2. Appl. Catal. A Gen. 2006, 310, 127–137. [Google Scholar] [CrossRef]
- Da Silva, R.J.; Pimentel, A.F.; Monteiro, R.S.; Mota, C. Synthesis of methanol and dimethyl ether from the CO2 hydrogenation over CuZnO supported on Al2O3 and Nb2O5. J. CO2 Util. 2016, 15, 83–88. [Google Scholar] [CrossRef]
- Zabidi, N.A.M.; Tasfy, S.; Shaharun, M.S. Effects of Nb Promoter on the Properties of Cu/ZnO/SBA-15 Catalyst and Performance in Methanol Production. Key Eng. Mater. 2016, 708, 94–97. [Google Scholar] [CrossRef]
- Saito, M.; Fujitani, T.; Takeuchi, M.; Watanabe, T. Development of copper/zinc oxide-based multicomponent catalysts for methanol synthesis from carbon dioxide and hydrogen. Appl. Catal. A Gen. 1996, 138, 311–318. [Google Scholar] [CrossRef]
- Toyir, J.; Piscina, P.R.; Fierro, J.L.G.; Homsa, N. Highly effective conversion of CO2 to methanol over supported and promoted copper-based catalysts: Influence of support and promoter. Appl. Catal. B Environ. 2001, 29, 207–215. [Google Scholar] [CrossRef]
- Li, M.M.-J.; Zeng, Z.; Liao, F.; Hong, X.; Tsang, S.C.E. Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts. J. Catal. 2016, 343, 157–167. [Google Scholar] [CrossRef]
- Tripathi, K.; Gupta, V.; Pant, K.K.; Upadhyayula, S. Deciphering Mn modulated structure-activity interplay and rational sta-tistical analysis for CO2 rich syngas hydrogenation to clean methanol. J. Clean. Prod. 2022, 340, 130794. [Google Scholar] [CrossRef]
- Marcos, F.C.F.; Cavalcanti, F.M.; Petrolini, D.D.; Lin, L.; Betancourt, L.E.; Senanayake, S.D.; Rodriguez, J.A.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Effect of operating parameters on H2/CO2 conversion to methanol over Cu-Zn oxide supported on ZrO2 polymorph catalysts: Characterization and kinetics. Chem. Eng. J. 2022, 427, 130947. [Google Scholar] [CrossRef]
- Zhang, W.B.; Liu, J.; Lu, S.H.; Zhang, H.; Wang, H.; Wang, X.D.; Cao, Q.P.; Zhang, D.X.; Jiang, J.Z. Size effect on atomic structure in low-dimensional Cu-Zr amorphous systems. Sci. Rep. 2017, 7, 7291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, M.K.; Wong, Y.; Chai, S.P.; Mohamed, A.R. Carbon dioxide hydrogenation to methanol over multi-functional catalyst: Effects of reactants adsorption and metal-oxide(s) interfacial area. J. Ind. Eng. Chem. 2018, 62, 156–165. [Google Scholar] [CrossRef]
- Din, I.U.; Shaharun, M.; Subbarao, D.; Naeem, A.; Hussain, F. Influence of niobium on carbon nanofibres based Cu/ZrO2 catalysts for liquid phase hydrogenation of CO2 to methanol. Catal. Today 2016, 259, 303–311. [Google Scholar] [CrossRef]
- Lopez-Suarez, F.E.; Bueno-López, A.; Illán-Gómez, M. Cu/Al2O3 catalysts for soot oxidation: Copper loading effect. Appl. Catal. B Environ. 2008, 84, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhan, H.; Zhao, N.; Xiao, F. CO2 hydrogenation to methanol over La-Mn-Cu-Zn-O based catalysts derived from perovskite precursors. Int. J. Hydrogen Energy 2017, 42, 20649–20657. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Wei, W.; Sun, Y. Fluorine-modified Mg–Al mixed oxides: A solid base with variable basic sites and tunable basicity. Appl. Catal. A Gen. 2010, 377, 107–113. [Google Scholar] [CrossRef]
- Ren, S.; Fan, X.; Shang, Z.; Shoemaker, W.R.; Ma, L.; Wu, T.; Li, S.; Klinghoffer, N.B.; Yu, M.; Liang, X. Enhanced catalytic performance of Zr modified CuO/ZnO/Al2O3 catalyst for methanol and DME synthesis via CO2 hydrogenation. J. CO2 Util. 2019, 36, 82–95. [Google Scholar] [CrossRef]
- Trifan, B.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. Modifications in the composition of CuO/ZnO/Al2O3 catalyst for the synthesis of methanol by CO2 Hydrogenation. Catalysts 2021, 11, 774. [Google Scholar] [CrossRef]
- Tursunov, O.; Kustov, L.; Tilyabaev, Z. Methanol synthesis from the catalytic hydrogenation of CO2 over CuO–ZnO supported on aluminum and silicon oxides. J. Taiwan Inst. Chem. Eng. 2017, 78, 416–422. [Google Scholar] [CrossRef]





| Catalyst | Promoter | T (°C) | P (MPa) | GHSV * | CO2 Conv. (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Cu/ZnO/Al2O3 | - | 250 | 5 | 4000 h−1 | 20.2 | 42.3 | [18] |
| Cu/ZnO/Al2O3 | Zr(0.3) | 250 | 5 | 4000 h−1 | 22.5 | 47.4 | [18] |
| Cu/γ-Al2O3 | - | 250 | 2 | 1400 h−1 | 8.98 | 13.4 | [19] |
| Cu/γ-Al2O3 | ZnO, ZrO2, MgO | 250 | 2 | 1400 h−1 | 12.12 | 35.98 | [19] |
| Cu/ZnO/Al2O3 | - | 200 | 5 | 260 mL min−1 g−1 | 7.1 | 78 | [20] |
| Cu/ZnO/Al2O3 | MgO | 200 | 5 | 260 mL min−1 g−1 | 8.1 | 87 | [20] |
| Cu/ZnO/ZrO2 | - | 250 | 3 | 61.5 mL min−1 g−1 | 4.08 | 63.6 | [21] |
| Cu/ZnO/Al2O3 | - | 240 | 2 | 1350 h−1 | 4.2 | 25.8 | [22] |
| Cu/ZnO/Al2O3 | ZrO2 | 240 | 2 | 1350 h−1 | 7.4 | 58.1 | [22] |
| Cu/ZnO/Al2O3 | Ga2O3 | 240 | 2 | 1350 h−1 | 4.4 | 32.5 | [22] |
| Cu/ZnO/Al2O3 | - | 250 | 5 | 200 mL min−1 g−1 | 19.7 | 39.7 | [23] |
| Cu/ZnO/Al2O3 | Mn | 250 | 5 | 200 mL min−1 g−1 | 22.3 | 43 | [23] |
| Cu/ZnO/Al2O3 | Zr | 250 | 5 | 200 mL min−1 g−1 | 24.7 | 48 | [23] |
| Cu/ZnO | Al2O3 | 230 | 3 | - | 18.7 | 43 | [24] |
| Cu/ZnO | Al2O3- ZrO2 | 230 | 3 | - | 23.2 | 60.3 | [24] |
| Cu/ZnO/ZrO2 | Ga2O3 | 250 | 8 | 3300 h−1 | - | 75 | [25] |
| Cu/ZnO/Al2O3 | - | 250 | 5 | 10 h−1 | 4 | 76 | [26] |
| Cu/ZnO/Nb2O5 | - | 250 | 5 | 10 h−1 | 1 | 100 | [26] |
| Cu/ZnO/SBA15 | Nb | 250 | 2 | 175 mL min−1 g−1 | 17.1 | 98 | [27] |
| Catalyst | CuO and ZnO Particle Size (nm) | Standard Deviations (nm) |
|---|---|---|
| CZAZ (un-promoted) | 47.4 | 3.4 |
| Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 6.7 | 2.9 |
| Mn/2Nb/Cu/ZnO/Al2O3-ZrO2 | 51.4 | 2.8 |
| 2Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 34.9 | 3.0 |
| Samples | Total H2 Consumption (µmol/g) | Reduction Temperature (°C) | H2 Consumption (%) | ||
|---|---|---|---|---|---|
| α | β | [α/(α + β) × 100] | [β/(α + β) × 100] | ||
| CZAZ (un-promoted) | 885 | - | 315 | - | 100 |
| Nb/Cu/ZnO/Al2O3-ZrO2 | 790 | 237 | 263 | 41 | 59 |
| Mn/Cu/ZnO/Al2O3-ZrO2 | 907 | 238 | 277 | 23 | 77 |
| Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 1030 | 284 | 296 | 47 | 53 |
| Mn/2Nb/Cu/ZnO/Al2O3-ZrO2 | 919 | 235 | 275 | 53 | 47 |
| 2Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 1243 | 243 | 281 | 34 | 66 |
| Samples | Total Basic Sites (µmol/g) | Peak Temperature (°C) | Number of Basic Sites (µmol/g) | ||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | α | β | γ | ||
| CZAZ (un-promoted) | 171 | 136 | 392 | 893 | 68 | 10 | 92 |
| Nb/Cu/ZnO/Al2O3-ZrO2 | 301 | 140 | 391 | 867 | 86 | 78 | 137 |
| Mn/Cu/ZnO/Al2O3-ZrO2 | 207 | 137 | 339 | 875 | 76 | 36 | 96 |
| Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 151 | 138 | 407 | 834 | 36 | 87 | 29 |
| Mn/2Nb/Cu/ZnO/Al2O3-ZrO2 | 137 | 136 | 357 | 881 | 38 | 27 | 71 |
| 2Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 163 | 141 | 368 | 863 | 79 | 39 | 45 |
| Sample | Promoter | SBET (m2/g) | Vp (cm3/g) | DBJH (nm) |
|---|---|---|---|---|
| Al2O3-ZrO2 (support) | - | 182 | 0.38 | 8.30 |
| CZAZ (un-promoted) | - | 154 | 0.33 | 6.13 |
| Nb/Cu/ZnO/Al2O3-ZrO2 | Nb | 134 | 0.30 | 6.50 |
| Mn/Cu/ZnO/Al2O3-ZrO2 | Mn | 162 | 0.36 | 6.50 |
| Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | Mn:Nb | 140 | 0.31 | 6.18 |
| Mn/2Nb/Cu/ZnO/Al2O3-ZrO2 | Mn:2Nb | 162 | 0.35 | 6.30 |
| 2Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 2Mn:Nb | 163 | 0.35 | 6.30 |
| Samples | Weight (%) | |||
|---|---|---|---|---|
| Cu | Zn | Mn | Nb | |
| Al2O3-ZrO2 (support) | - | - | - | - |
| CZAZ (un-promoted) | 11.24 | 3.85 | - | - |
| Nb/Cu/ZnO/Al2O3-ZrO2 | 11.41 | 3.61 | - | 0.08 |
| Mn/Cu/ZnO/Al2O3-ZrO2 | 11.17 | 3.84 | 0.08 | - |
| Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 11.27 | 3.72 | 0.05 | 0.04 |
| Mn/2Nb/Cu/ZnO/Al2O3-ZrO2 | 11.45 | 3.55 | 0.03 | 0.05 |
| 2Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 11.28 | 3.71 | 0.04 | 0.06 |
| Total: ~15.09% | ||||
| Catalyst | Promoter Ratio | Cu Surface Area, SCu (m2/g) | Cu Dispersion DCu (%) | Cu Particle Size, dCu (nm) |
|---|---|---|---|---|
| CZAZ | Un-promoted | 6.00 | 8.87 | 9.80 |
| Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 1:1 | 1.87 | 2.77 | 31.3 |
| Mn/2Nb/Cu/ZnO/Al2O3-ZrO2 | 1:2 | 5.66 | 8.37 | 10.3 |
| 2Mn/Nb/Cu/ZnO/Al2O3-ZrO2 | 2:1 | 6.41 | 9.48 | 9.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkifli, N.I.; Zabidi, N.A.M.; Merican, Z.M.A.; Tasfy, S.F.H. Effects of Promoter’s Composition on the Physicochemical Properties of Cu/ZnO/Al2O3-ZrO2 Catalyst. Catalysts 2022, 12, 636. https://doi.org/10.3390/catal12060636
Zulkifli NI, Zabidi NAM, Merican ZMA, Tasfy SFH. Effects of Promoter’s Composition on the Physicochemical Properties of Cu/ZnO/Al2O3-ZrO2 Catalyst. Catalysts. 2022; 12(6):636. https://doi.org/10.3390/catal12060636
Chicago/Turabian StyleZulkifli, Nur Insyirah, Noor Asmawati Mohd Zabidi, Zulkifli Merican Aljunid Merican, and Sara Faiz Hanna Tasfy. 2022. "Effects of Promoter’s Composition on the Physicochemical Properties of Cu/ZnO/Al2O3-ZrO2 Catalyst" Catalysts 12, no. 6: 636. https://doi.org/10.3390/catal12060636
APA StyleZulkifli, N. I., Zabidi, N. A. M., Merican, Z. M. A., & Tasfy, S. F. H. (2022). Effects of Promoter’s Composition on the Physicochemical Properties of Cu/ZnO/Al2O3-ZrO2 Catalyst. Catalysts, 12(6), 636. https://doi.org/10.3390/catal12060636