CO Oxidation over Alumina-Supported Copper Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Properties
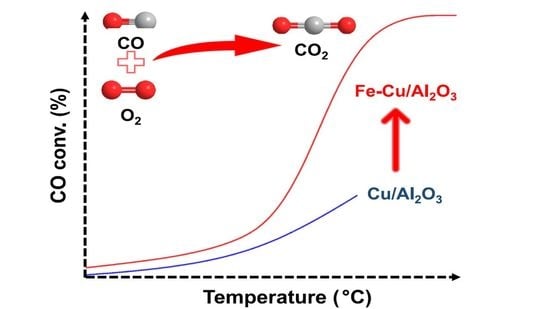
2.2. Catalytic Evaluation of the CO Oxidation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. X-Ray Absorption Spectroscopy Measurement
3.4. Catalytic Evaluation of CO Oxidation
3.5. Carbon Monoxide Temperature-Programmed Reduction (CO-TPR)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Surface science under reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev. 2017, 46, 4347–4374. [Google Scholar] [CrossRef] [PubMed]
- Stamenković, V.; Arenz, M.; Blizanac, B.; Mayrhofer, K.; Ross, P.; Marković, N. In situ CO oxidation on well characterized Pt3Sn (hkl) surfaces: A selective review. Surf. Sci. 2005, 576, 145–157. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Z.; Jia, P. Pd-doped C3N monolayer: A promising low-temperature and high-activity single-atom catalyst for CO oxidation. Appl. Surf. Sci. 2020, 537, 147881. [Google Scholar] [CrossRef]
- Meunier, F.C.; Cardenas, L.; Kaper, H.; Šmíd, B.; Vorokhta, M.; Grosjean, R.; Aubert, D.; Dembélé, K.; Lunkenbein, T. Synergy between metallic and oxidized Pt sites unravelled during room temperature CO oxidation on Pt/ceria. Angew. Chem. Int. Ed. 2021, 60, 3799–3805. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R.J. Activation of Molecular Oxygen and the Nature of the Active Oxygen Species for CO Oxidation on Oxide Supported Au Catalysts. Accounts Chem. Res. 2014, 47, 740–749. [Google Scholar] [CrossRef]
- Min, B.K.; Friend, C.M. Heterogeneous Gold-Based Catalysis for Green Chemistry: Low-Temperature CO Oxidation and Propene Oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chin. J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Xu, S.; Chansai, S.; Xu, S.; Stere, C.E.; Jiao, Y.; Yang, S.; Hardacre, C.; Fan, X. CO poisoning of Ru catalysts in CO2 hydrogenation under thermal and plasma conditions: A combined kinetic and diffuse reflectance infrared fourier transform spectroscopy–mass spectrometry study. ACS Catal. 2020, 10, 12828–12840. [Google Scholar] [CrossRef]
- Knudsen, J.; Nilekar, A.U.; Vang, R.T.; Schnadt, J.; Kunkes, E.L.; Dumesic, J.A.; Mavrikakis, M.; Besenbacher, F. A Cu/Pt Near-Surface Alloy for Water-Gas Shift Catalysis. J. Am. Chem. Soc. 2007, 129, 6485–6490. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Wang, X.; Mei, B.; Luo, E.; Li, Y.; Meng, Q.; Jin, Z.; Jiang, Z.; Liu, C.; et al. CO-Tolerant PEMFC Anodes Enabled by Synergistic Catalysis between Iridium Single-Atom Sites and Nanoparticles. Angew. Chem. 2021, 133, 26381–26387. [Google Scholar] [CrossRef]
- Mosrati, J.; Abdel-Mageed, A.M.; Vuong, T.H.; Grauke, R.; Bartling, S.; Rockstroh, N.; Atia, H.; Armbruster, U.; Wohlrab, S.; Rabeah, J.; et al. Tiny species with big impact: High activity of Cu single atoms on CeO2–TiO2 deciphered by operando spectroscopy. ACS Catal. 2021, 11, 10933–10949. [Google Scholar] [CrossRef]
- Huang, T.-J.; Tsai, D.-H. CO oxidation behavior of copper and copper oxides. Catal. Lett. 2003, 87, 173–178. [Google Scholar] [CrossRef]
- Falsig, H.; Hvolbæk, B.; Kristensen, I.S.; Jiang, T.; Bligaard, T.; Christensen, C.H.; Nørskov, J.K. Trends in the Catalytic CO Oxidation Activity of Nanoparticles. Angew. Chem. 2008, 120, 4913–4917. [Google Scholar] [CrossRef]
- Jia, A.-P.; Hu, G.-S.; Meng, L.; Xie, Y.-L.; Lu, J.-Q.; Luo, M.-F. CO oxidation over CuO/Ce1−xCuxO2−δ and Ce1−xCuxO2−δ catalysts: Synergetic effects and kinetic study. J. Catal. 2012, 289, 199–209. [Google Scholar] [CrossRef]
- Zhang, X.-m.; Tian, P.; Tu, W.; Zhang, Z.; Xu, J.; Han, Y.-F. Tuning the dynamic interfacial structure of copper–ceria catalysts by indium oxide during CO oxidation. ACS Catal. 2018, 8, 5261–5275. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Hu, P.; Alavi, A. Mechanism for the high reactivity of CO oxidation on a ruthenium–oxide. J. Chem. Phys. 2001, 114, 5956–5957. [Google Scholar] [CrossRef]
- Huang, B.; Kobayashi, H.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Nishida, Y.; Sato, K.; Nagaoka, K.; Haneda, M.; Xie, W.; et al. A CO adsorption site change induced by copper substitution in a ruthenium catalyst for enhanced CO oxidation activity. Angew. Chem. Int. Ed. 2019, 131, 2252–2257. [Google Scholar] [CrossRef]
- Kim, Y.; Over, H.; Krabbes, G.; Ertl, G. Identification of RuO2 as the active phase in CO oxidation on oxygen-rich ruthenium surfaces. Top. Catal. 2000, 14, 95–100. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Renzas, J.; Butcher, D.R.; Huang, W.; Somorjai, G.A. Size Effect of Ruthenium Nanoparticles in Catalytic Carbon Monoxide Oxidation. Nano Lett. 2010, 10, 2709–2713. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, A.; Saraev, A.; Kremneva, A.; Selivanova, A.; Vorokhta, M.; Šmíd, B.; Bulavchenko, O.; Yakovlev, V.; Kaichev, V. Kinetic and mechanistic study of CO oxidation over nanocomposite Cu-Fe-Al oxide catalysts. ChemCatChem 2020, 12, 4911–4921. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Tsapina, A.M.; Bulavchenko, O.A.; Saraev, A.A.; Odegova, G.V.; Ermakov, D.Y.; Zubavichus, Y.V.; Yakovlev, V.A.; Kaichev, V.V. Structure and chemistry of Cu–Fe–Al nanocomposite catalysts for CO oxidation. Catal. Lett. 2018, 148, 3715–3722. [Google Scholar] [CrossRef]
- Engel, T.; Ertl, G. Surface residence times and reaction mechanism in the catalytic oxidation of CO on Pd(111). Chem. Phys. Lett. 1978, 54, 95–98. [Google Scholar] [CrossRef]
- Cisternas, J.; Holmes, P.; Kevrekidis, I.G.; Li, X. CO oxidation on thin Pt crystals: Temperature slaving and the derivation of lumped models. J. Chem. Phys. 2003, 118, 3312–3328. [Google Scholar] [CrossRef]
- Baxter, R.; Hu, P. Insight into why the Langmuir–Hinshelwood mechanism is generally preferred. J. Chem. Phys. 2002, 116, 4379–4381. [Google Scholar] [CrossRef]
- Hopstaken, M.J.P.; Niemantsverdriet, J.W. Structure sensitivity in the CO oxidation on rhodium: Effect of adsorbate coverages on oxidation kinetics on Rh(100) and Rh(111). J. Chem. Phys. 2000, 113, 5457. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R. Dynamic surface composition in a Mars-van Krevelen type reaction: CO oxidation on Au/TiO2. J. Catal. 2018, 357, 263–273. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.; Liu, L.; Zhang, H.; Gao, F.; Dong, L.; Chen, Y. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl. Catal. B 2012, 119, 308–320. [Google Scholar] [CrossRef]
- Niu, J.; Liland, S.E.; Yang, J.; Rout, K.R.; Ran, J.; Chen, D. Effect of oxide additives on the hydrotalcite derived Ni catalysts for CO2 reforming of methane. Chem. Eng. J. 2018, 377, 119763. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Ma, H.; Sollund, E.S.; Zhang, W.; Fenes, E.; Qi, Y.; Wang, Y.; Rout, K.R.; Fuglerud, T.; Piccinini, M.; Chen, D. Kinetic modeling of dynamic changing active sites in a Mars-van Krevelen type reaction: Ethylene oxychlorination on K-doped CuCl2/Al2O3. Chem. Eng. J. 2021, 407, 128013. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Zhang, H.; Ma, G.; Zhang, W.; Qi, Y.; Fuglerud, T.; Jiang, Z.; Ding, W.; Chen, D. Facet-Induced Strong Metal Chloride-Support Interaction over CuCl2/γ-Al2O3 Catalyst to Enhance Ethylene Oxychlorination Performance. ACS Catal. 2022, 12, 8027–8037. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Qi, Y.; Rout, K.R.; Chen, D. Critical Review of Catalysis for Ethylene Oxychlorination. ACS Catal. 2020, 10, 9299–9319. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Tang, Y.-Q. Spontaneous monolayer dispersion of oxides and salts onto surfaces of supports: Applications to heterogeneous catalysis. Adv. Catal. 1990, 37, 1–43. [Google Scholar] [CrossRef]
- Ma, H.; Fenes, E.; Qi, Y.; Wang, Y.; Rout, K.R.; Fuglerud, T.; Chen, D. Understanding of K and Mg co-promoter effect in ethylene oxychlorination by operando UV–vis-NIR spectroscopy. Catal. Today 2020, 369, 227–234. [Google Scholar] [CrossRef]
- Yang, G.; Haibo, Z.; Biying, Z. Monolayer dispersion of oxide additives on SnO2 and their promoting effects on thermal stability of SnO2 ultrafine particles. J. Mater. Sci. 2000, 35, 917–923. [Google Scholar] [CrossRef]
- Yu, X.-F.; Wu, N.-Z.; Xie, Y.-C.; Tang, Y.-Q. A monolayer dispersion study of titania-supported copper oxide. J. Mater. Chem. 2000, 10, 1629–1634. [Google Scholar] [CrossRef]
- Jernigan, G.G.; Somorjai, G.A. Carbon monoxide oxidation over three different oxidation states of copper: Metallic copper, copper (I) oxide, and copper (II) oxide-a surface science and kinetic study. J. Catal. 1994, 147, 567–577. [Google Scholar] [CrossRef]
- Nagase, K.; Zheng, Y.; Kodama, Y.; Kakuta, J. Dynamic Study of the Oxidation State of Copper in the Course of Carbon Monoxide Oxidation over Powdered CuO and Cu2O. J. Catal. 1999, 187, 123–130. [Google Scholar] [CrossRef]







| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Metal Loading (wt%) |
|---|---|---|---|---|
| Cu/γ-Al2O3 | 181 | 0.55 | 9.07 | 5 |
| FeCu/γ-Al2O3 | 144 | 0.43 | 9.10 | 1/5 (Fe/Cu) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Wang, L.; Wang, X.; Li, L.; Ma, H. CO Oxidation over Alumina-Supported Copper Catalysts. Catalysts 2022, 12, 1030. https://doi.org/10.3390/catal12091030
Ma G, Wang L, Wang X, Li L, Ma H. CO Oxidation over Alumina-Supported Copper Catalysts. Catalysts. 2022; 12(9):1030. https://doi.org/10.3390/catal12091030
Chicago/Turabian StyleMa, Guoyan, Le Wang, Xiaorong Wang, Lu Li, and Hongfei Ma. 2022. "CO Oxidation over Alumina-Supported Copper Catalysts" Catalysts 12, no. 9: 1030. https://doi.org/10.3390/catal12091030
APA StyleMa, G., Wang, L., Wang, X., Li, L., & Ma, H. (2022). CO Oxidation over Alumina-Supported Copper Catalysts. Catalysts, 12(9), 1030. https://doi.org/10.3390/catal12091030