Modification of Graphite Sheet Anode with Iron (II, III) Oxide-Carbon Dots for Enhancing the Performance of Microbial Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Properties
2.1.1. X-ray Diffraction
2.1.2. Scanning Electron Microscopy (SEM)
2.1.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.4. RAMAN Spectroscopy
2.2. Optical Properties
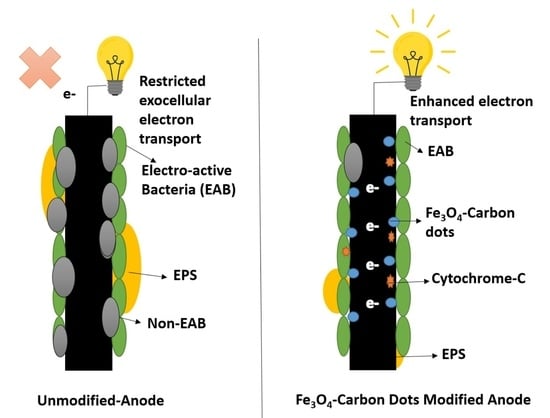
2.3. Microbial Electrochemical Study
2.4. Power Generation
3. Materials and Methods
3.1. Synthesis of Fe-Carbon Dots
3.2. Characterization of Fe-Carbon Dots
3.3. Construction of Electrodes
3.4. MFC Fabrication and Operation
3.5. Computation and Analysis
3.6. Analyses of Improved Anodes
3.7. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Tian, X.; Xiao, Y.; Ulstrup, J.; Christensen, H.E.M.; Zhao, F.; Zhang, J. Selective Electrocatalysis of Biofuel Molecular Oxidation Using Palladium Nanoparticles Generated on Shewanella Oneidensis MR-1. J. Mater. Chem. A 2018, 6, 10655–10662. [Google Scholar] [CrossRef]
- Rojas Flores, S.; Nazario-Naveda, R.; Betines, S.; Cruz–Noriega, M.; Cabanillas-Chirinos, L.; Valdiviezo Dominguez, F. Sugar Industry Waste for Bioelectricity Generation. Environ. Res. Eng. Manag. 2021, 77, 15–22. [Google Scholar] [CrossRef]
- Chou, H.-T.; Lee, H.-J.; Lee, C.-Y.; Tai, N.-H.; Chang, H.-Y. Highly Durable Anodes of Microbial Fuel Cells Using a Reduced Graphene Oxide/Carbon Nanotube-Coated Scaffold. Bioresour. Technol. 2014, 169, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Multi-Walled Carbon Nanotube/SnO2 Nanocomposite: A Novel Anode Material for Microbial Fuel Cells. Electrochim. Acta 2014, 130, 512–518. [Google Scholar] [CrossRef]
- Min, B.; Román, O.B.; Angelidaki, I. Importance of Temperature and Anodic Medium Composition on Microbial Fuel Cell (MFC) Performance. Biotechnol. Lett. 2008, 30, 1213–1218. [Google Scholar] [CrossRef]
- Torres, C.I.; Krajmalnik-Brown, R.; Parameswaran, P.; Marcus, A.K.; Wanger, G.; Gorby, Y.A.; Rittmann, B.E. Selecting Anode-Respiring Bacteria Based on Anode Potential: Phylogenetic, Electrochemical, and Microscopic Characterization. Environ. Sci. Technol. 2009, 43, 9519–9524. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, S.; Schaller, R.; Jiao, J.; Chaplen, F.; Liu, H. Nanoparticle Decorated Anodes for Enhanced Current Generation in Microbial Electrochemical Cells. Biosens. Bioelectron. 2011, 26, 1908–1912. [Google Scholar] [CrossRef]
- Neethu, B.; Bhowmick, G.D.; Ghangrekar, M.M. Improving Performance of Microbial Fuel Cell by Enhanced Bacterial-Anode Interaction Using Sludge Immobilized Beads with Activated Carbon. Process. Saf. Environ. Prot. 2020, 143, 285–292. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Noori, M.D.T.; Das, I.; Neethu, B.; Ghangrekar, M.M.; Mitra, A. Bismuth Doped TiO2 as an Excellent Photocathode Catalyst to Enhance the Performance of Microbial Fuel Cell. Int. J. Hydrogen Energy 2018, 43, 7501–7510. [Google Scholar] [CrossRef]
- Ahilan, V.; Bhowmick, G.D.; Ghangrekar, M.M.; Wilhelm, M.; Rezwan, K. Tailoring Hydrophilic and Porous Nature of Polysiloxane Derived Ceramer and Ceramic Membranes for Enhanced Bioelectricity Generation in Microbial Fuel Cell. Ionics 2019, 25, 5907–5918. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, S.; Guo, J. Anode Modification with Formic Acid: A Simple and Effective Method to Improve the Power Generation of Microbial Fuel Cells. Appl. Surf. Sci. 2014, 320, 281–286. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ye, D.; Zhu, X.; Liao, Q.; Zhang, B. Enhanced Performances of Microbial Fuel Cells Using Surface-Modified Carbon Cloth Anodes: A Comparative Study. Int. J. Hydrogen Energy 2014, 39, 19148–19155. [Google Scholar] [CrossRef]
- Rajesh, P.P.; Noori, M.D.T.; Ghangrekar, M.M. Controlling Methanogenesis and Improving Power Production of Microbial Fuel Cell by Lauric Acid Dosing. Water Sci. Technol. 2014, 70, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.P.; Jadhav, D.A.; Ghangrekar, M.M. Improving Performance of Microbial Fuel Cell While Controlling Methanogenesis by Chaetoceros Pretreatment of Anodic Inoculum. Bioresour. Technol. 2015, 180, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, H.; Du, Z.; Ng, H.Y. Spontaneous Modification of Graphite Anode by Anthraquinone-2-Sulfonic Acid for Microbial Fuel Cells. Bioresour. Technol. 2014, 164, 184–188. [Google Scholar] [CrossRef]
- Park, D.H.; Laivenieks, M.; Guettler, M.V.; Jain, M.K.; Zeikus, J.G. Microbial Utilization of Electrically Reduced Neutral Red as the Sole Electron Donor for Growth and Metabolite Production. Appl. Env. Microbiol. 1999, 65, 2912–2917. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.; Ittitanakam, A.; Tanubumrungsuk, W.; Chaithong, S.; Thanosawan, S.; Sinbuathong, N.; Jeraputra, C. Potential of Lignin as a Mediator in Combined Systems for Biomethane and Electricity Production from Ethanol Stillage Wastewater. Renew. Energy 2015, 76, 242–248. [Google Scholar] [CrossRef]
- Vishwanathan, A.S.; Aiyer, K.S.; Chunduri, L.A.A.; Venkataramaniah, K.; Siva Sankara Sai, S.; Rao, G. Carbon Quantum Dots Shuttle Electrons to the Anode of a Microbial Fuel Cell. 3 Biotech 2016, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.W.; Soomro, R.A.; Kalwar, N.H.; Zahoor, M.; Avci, A.; Pehlivan, E.; Hallam, K.R.; Willander, M. Carbon Quantum Dot Coated Fe3O4 Hybrid Composites for Sensitive Electrochemical Detection of Uric Acid. Microchem. J. 2019, 146, 517–524. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Z.; Matsui, H.; Zhou, S. Fe3O4/Carbon Quantum Dots Hybrid Nanoflowers for Highly Active and Recyclable Visible-Light Driven Photocatalyst. J. Mater. Chem. A 2014, 2, 15740–15745. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, M.; Yousuf, A.; Rathi, P.; Sahni, M.; Singh, S. Effect of Na/Co Co-Substituted on Structural, Magnetic, Optical and Photocatalytic Properties of BiFeO3 Nanoparticles. Mater. Chem. Phys. 2021, 263, 124402. [Google Scholar] [CrossRef]
- Lemine, O.M. Transformation of Goethite to Hematite Nanocrystallines by High Energy Ball Milling. Adv. Mater. Sci. Eng. 2014, 2014, e589146. [Google Scholar] [CrossRef]
- Periyasamy, M.; Sain, S.; Sengupta, U.; Mandal, M.; Mukhopadhyay, S.; Kar, A. Bandgap Tuning of Photo Fenton-like Fe3O4/C Catalyst through Oxygen Vacancies for Advanced Visible Light Photocatalysis. Mater. Adv. 2021, 2, 4843–4858. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.S. Green Synthesis of Luminescent Nitrogen-Doped Carbon Dots from Milk and Its Imaging Application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal Green Synthesis of Magnetic Fe3O4-Carbon Dots by Lemon and Grape Fruit Extracts and as a Photoluminescence Sensor for Detecting of E. Coli Bacteria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 481–493. [Google Scholar] [CrossRef]
- Kumar, G.G.; Sarathi, V.G.S.; Nahm, K.S. Recent Advances and Challenges in the Anode Architecture and Their Modifications for the Applications of Microbial Fuel Cells. Biosens. Bioelectron. 2013, 43, 461–475. [Google Scholar] [CrossRef]
- Fricke, K.; Harnisch, F.; Schröder, U. On the Use of Cyclic Voltammetry for the Study of Anodic Electron Transfer in Microbial Fuel Cells. Energy Environ. Sci. 2008, 1, 144–147. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Baranitharan, E.; Khan, M.R.; Prasad, D.M.R.; Teo, W.F.A.; Tan, G.Y.A.; Jose, R. Effect of Biofilm Formation on the Performance of Microbial Fuel Cell for the Treatment of Palm Oil Mill Effluent. Bioprocess. Biosyst. Eng. 2015, 38, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhou, S.; Zhao, B.; Zhuang, L.; Wang, Y. Microbially-Reduced Graphene Scaffolds to Facilitate Extracellular Electron Transfer in Microbial Fuel Cells. Bioresour. Technol. 2012, 116, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Khilari, S.; Roy, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Reduction of Start-up Time through Bioaugmentation Process in Microbial Fuel Cells Using an Isolate from Dark Fermentative Spent Media Fed Anode. Water Sci. Technol. 2015, 72, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An Overview of Electrode Materials in Microbial Fuel Cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Neto, D.M.A.; Freire, R.M.; Gallo, J.; Freire, T.M.; Queiroz, D.C.; Ricardo, N.M.P.S.; Vasconcelos, I.F.; Mele, G.; Carbone, L.; Mazzetto, S.E.; et al. Rapid Sonochemical Approach Produces Functionalized Fe3O4 Nanoparticles with Excellent Magnetic, Colloidal, and Relaxivity Properties for MRI Application. J. Phys. Chem. C 2017, 121, 24206–24222. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Das, D.; Pradhan, D. Graphene Supported α-MnO 2 Nanotubes as a Cathode Catalyst for Improved Power Generation and Wastewater Treatment in Single-Chambered Microbial Fuel Cells. RSC Adv. 2013, 3, 7902–7911. [Google Scholar] [CrossRef]
- Panda, J.; Chowdhury, R. Growth Kinetic Study of Electrochemically Active Bacterium Shewanella Putrefaciens MTCC 8104 on Acidic Effluent of Jute Stick Pyrolysis. Indian Chem. Eng. 2021, 63, 193–205. [Google Scholar] [CrossRef]
- More, T.T.; Ghangrekar, M.M. Improving Performance of Microbial Fuel Cell with Ultrasonication Pre-Treatment of Mixed Anaerobic Inoculum Sludge. Bioresour. Technol. 2010, 101, 562–567. [Google Scholar] [CrossRef]
- Behera, M.; Ghangrekar, M.M. Performance of Microbial Fuel Cell in Response to Change in Sludge Loading Rate at Different Anodic Feed PH. Bioresour. Technol. 2009, 100, 5114–5121. [Google Scholar] [CrossRef]
- Pandit, S.; Khilari, S.; Roy, S.; Pradhan, D.; Das, D. Improvement of Power Generation Using Shewanella Putrefaciens Mediated Bioanode in a Single Chambered Microbial Fuel Cell: Effect of Different Anodic Operating Conditions. Bioresour. Technol. 2014, 166, 451–457. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Varanasi, J.L.; Das, D.; Pradhan, D. Bifunctional Manganese Ferrite/Polyaniline Hybrid as Electrode Material for Enhanced Energy Recovery in Microbial Fuel Cell. ACS Appl. Mater. Interfaces 2015, 7, 20657–20666. [Google Scholar] [CrossRef]








| MFC Serial No. | Anode | OCV (mV) | PD (mW/m2) | Charge Transfer Resistance, Rct (Ohm) |
|---|---|---|---|---|
| MFC-1 | Raw graphite sheet | 701 | 285.01 | 230 |
| MFC-2 | Fe3O4@PSA-C modified anode (0.25 mg/cm2) | 726 | 334.41 | 164.46 |
| MFC-3 | Fe3O4@PSA-C modified anode (0.50 mg/cm2) | 746 | 379.56 | 103.14 |
| MFC-4 | Fe3O4@PSA-C modified anode (0.75 mg/cm2) | 765 | 420.33 | 52.96 |
| MFC-5 | Fe3O4@PSA-C modified anode (1 mg/cm2) | 771 | 440.01 | 33.45 |
| Packaging Type | Poly Packet |
|---|---|
| Brand | Amul |
| Serving Size | 200 mL/100 mL |
| Amount per | 100 mL/100 mL |
| Energy | 62 Kcal/100 mL |
| Energy from FAT | 32 Kcal/100 mL |
| Total FAT | 3.5 g/100 mL |
| Saturated FAT | 2.3 g/100 mL |
| Cholesterol | 8 mg/100 mL |
| Total Carbohydrate | 4.7 g/100 mL |
| Pack Size | 500 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, B.; Pandit, S.; Sharma, A.; Chauhan, S.; Mathuriya, A.S.; Dikshit, P.K.; Gupta, P.K.; Singh, R.C.; Sahni, M.; Pant, K.; et al. Modification of Graphite Sheet Anode with Iron (II, III) Oxide-Carbon Dots for Enhancing the Performance of Microbial Fuel Cell. Catalysts 2022, 12, 1040. https://doi.org/10.3390/catal12091040
Tripathi B, Pandit S, Sharma A, Chauhan S, Mathuriya AS, Dikshit PK, Gupta PK, Singh RC, Sahni M, Pant K, et al. Modification of Graphite Sheet Anode with Iron (II, III) Oxide-Carbon Dots for Enhancing the Performance of Microbial Fuel Cell. Catalysts. 2022; 12(9):1040. https://doi.org/10.3390/catal12091040
Chicago/Turabian StyleTripathi, Babita, Soumya Pandit, Aparna Sharma, Sunil Chauhan, Abhilasha Singh Mathuriya, Pritam Kumar Dikshit, Piyush Kumar Gupta, Ram Chandra Singh, Mohit Sahni, Kumud Pant, and et al. 2022. "Modification of Graphite Sheet Anode with Iron (II, III) Oxide-Carbon Dots for Enhancing the Performance of Microbial Fuel Cell" Catalysts 12, no. 9: 1040. https://doi.org/10.3390/catal12091040
APA StyleTripathi, B., Pandit, S., Sharma, A., Chauhan, S., Mathuriya, A. S., Dikshit, P. K., Gupta, P. K., Singh, R. C., Sahni, M., Pant, K., & Singh, S. (2022). Modification of Graphite Sheet Anode with Iron (II, III) Oxide-Carbon Dots for Enhancing the Performance of Microbial Fuel Cell. Catalysts, 12(9), 1040. https://doi.org/10.3390/catal12091040