Embedding Thiophene-Amide into g-C3N4 Skeleton with Induction and Delocalization Effects for High Photocatalytic H2 Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Structure Analyses
2.2. Optical and Photoelectrochemical Properties Analyses
2.3. Band Structure Analyses and DFT Calculation
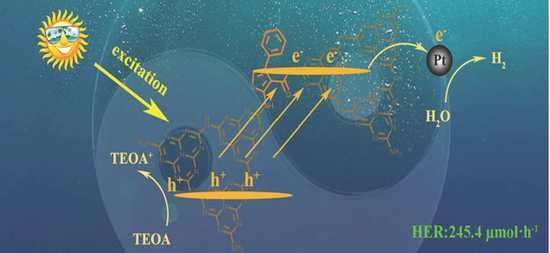
2.4. Photocatalytic Hydrogen Production Performance Analyses
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Photocatalysts
3.3. Characterization
3.4. Photoelectrochemical Test
3.5. Photocatalytic Hydrogen Evolution Test
3.6. Density Functional Theory (DFT) Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Mao, S.; Shi, M.; Hong, X.; Wang, D.; Wang, F.; Xia, M.; Chen, Q. Enhanced photocatalytic degradation performance of BiVO4/BiOBr through combining fermi level alteration and oxygen defect engineering. Chem. Eng. J. 2022, 449, 137757. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2021, 430, 132806. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Chen, W.; Lee, J.E.; Jang, S.; Lam, S.; Rhee, G.H.; Jeon, K.; Hussain, M.; Park, Y. A review on the visible light active modified photocatalysts for water splitting for hydrogen production. Int. J. Energy Res. 2021, 46, 5467–5477. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Pawar, R.C.; Kang, S.; Ahn, S.H.; Lee, C.S. Gold nanoparticle modified graphitic carbon nitride/multi-walled carbon nanotube (g-C3N4/CNTs/Au) hybrid photocatalysts for effective water splitting and degradation. RSC Adv. 2015, 5, 24281–24292. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Huang, W.; Kong, Q.; Fan, X.; Wang, M.; Shi, J. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H2 evolution under visible light. Carbon 2015, 99, 111–117. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Hou, L.-A. A critical review of g-C3N4-based photocatalytic membrane for water purification. Chem. Eng. J. 2021, 412, 128663. [Google Scholar] [CrossRef]
- Che, H.; Che, G.; Zhou, P.; Liu, C.; Dong, H.; Li, C.; Song, N.; Li, C. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2019, 382, 122870. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, H.; Yan, S.; Zou, Z. Synthesis of carbon black/carbon nitride intercalation compound composite for efficient hydrogen production. Dalton Trans. 2014, 43, 12013–12017. [Google Scholar] [CrossRef]
- Ho, W.; Zhang, Z.; Lin, W.; Huang, S.; Zhang, X.; Wang, X.; Huang, Y. Copolymerization with 2,4,6-triaminopyrimidine for the rolling-up the layer structure, tunable electronic properties, and photocatalysis of g-C3N4. ACS Appl. Mater. Interfaces 2015, 7, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.-S.; Yan, J.-M.; Wulan, B.-R.; Li, S.-J.; Liu, K.-H.; Jiang, Q. Noble-metal-free cobalt phosphide modified carbon nitride: An efficient photocatalyst for hydrogen generation. Appl. Catal. B Environ. 2017, 200, 477–483. [Google Scholar] [CrossRef]
- Ye, S.; Qiu, L.-G.; Yuan, Y.-P.; Zhu, Y.-J.; Xia, J.; Zhu, J.-F. Facile fabrication of magnetically separable graphitic carbon nitride photocatalysts with enhanced photocatalytic activity under visible light. J. Mater. Chem. A 2013, 1, 3008–3015. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Cheng, R.; Wang, M.; Li, M.; Zhou, Y.; Shi, J. Construction of Graphitic C3N4-Based Intramolecular Donor–Acceptor Conjugated Copolymers for Photocatalytic Hydrogen Evolution. ACS Catal. 2015, 5, 5008–5015. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; Liang, S.; Liu, M.; Bi, J.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Varma, S.; Tripathi, A.K.; Bharadwaj, S.R.; Tyagi, A.K. Effect of vanadia doping and its oxidation state on the photocatalytic activity of TiO2 for gas-phase oxidation of ethene. J. Phys. Chem. C 2008, 112, 19102–19112. [Google Scholar] [CrossRef]
- Gu, Q.; Liao, Y.; Yin, L.; Long, J.; Wang, X.; Xue, C. Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability. Appl. Catal. B Environ. 2015, 165, 503–510. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Wang, Z.; Zhang, X.; Zou, J.-J.; Mi, W.; Zhang, X.; Wang, L. Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 2015, 12, 646–656. [Google Scholar] [CrossRef]
- Dong, G.; Zhao, K.; Zhang, L. Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4. Chem. Commun. 2012, 48, 6178–6180. [Google Scholar] [CrossRef]
- Lu, C.; Chen, R.; Wu, X.; Fan, M.; Liu, Y.; Le, Z.; Jiang, S.; Song, S. Boron doped g-C3N4 with enhanced photocatalytic UO22+ reduction performance. Appl. Surf. Sci. 2016, 360, 1016–1022. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.M.; Cheng, H.-M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef]
- Bian, S.-W.; Ma, Z.; Song, W.-G. Preparation and characterization of carbon nitride nanotubes and their applications as catalyst supporter. J. Phys. Chem. C 2009, 113, 8668–8672. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C 3 N 4 nanosheets with highly efficient photocatalytic H 2 evolution from water under visible light. Appl. Catal. B Environ. 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Zhou, Y.; Wang, Y.; Qiu, K.; Zhang, C.; Fang, J.; Sheng, X. Facile one-step synthesis of hollow mesoporous g-C3N4 spheres with ultrathin nanosheets for photoredox water splitting. Carbon 2018, 126, 247–256. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Wang, T.; Zhang, P.; Li, A.; Gong, J. Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J. Mater. Chem. A 2013, 2, 2885–2890. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.; Wang, L.; Yan, J.; Li, H.; Song, Y.; Huang, L.; Cai, G. The CNT modified white C3N4 composite photocatalyst with enhanced visible-light response photoactivity. Dalton Trans. 2013, 42, 7604–7613. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Lu, Y.; Chu, D.; Zhu, M.; Du, Y.; Yang, P. Exfoliated carbon nitride nanosheets decorated with NiS as an efficient noble-metal-free visible-light-driven photocatalyst for hydrogen evolution. Phys. Chem. Chem. Phys. 2015, 17, 17355–17361. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.; Menezes, P.W.; Kailasam, K.; Hollmann, D.; Schröder, M.; Thomas, A.; Brückner, A.; Driess, M. Nickel as a co-catalyst for photocatalytic hydrogen evolution on graphitic-carbon nitride (sg-CN): What is the nature of the active species? Chem. Commun. 2015, 52, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, B.; Li, L.; Peng, F.; Wang, H.; Yu, H.; Fang, Y. A carbon nitride/TiO2 nanotube array heterojunction visible-light photocatalyst: Synthesis, characterization, and photoelectrochemical properties. J. Mater. Chem. 2012, 22, 17900–17905. [Google Scholar] [CrossRef]
- Li, K.; Zhang, W.-D. Creating graphitic carbon nitride based donor-π-acceptor-π-donor structured catalysts for highly photocatalytic hydrogen evolution. Small 2018, 14, 1703599. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Zhang, M.; Wang, X. Molecular and textural engineering of conjugated carbon nitride catalysts for selective oxidation of alcohols with visible light. Chem. Sci. 2013, 4, 3244–3248. [Google Scholar] [CrossRef]
- Heymann, L.; Bittinger, S.C.; Klinke, C. Molecular doping of electrochemically prepared triazine-based carbon nitride by 2,4,6-triaminopyrimidine for improved photocatalytic properties. ACS Omega 2018, 3, 17042–17048. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Shanmugam, R.; Gangavarapu, R.R. Extending the π-electron conjugation in 2D planar graphitic carbon nitride: Efficient charge separation for overall water splitting. J. Mater. Chem. A 2019, 7, 3757–3771. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Zhu, D.; Zhou, T.; Yan, M.; Chen, G.; Sun, J.; Dai, G.; Ge, F.; Dong, H. High-efficient charge separation driven directionally by pyridine rings grafted on carbon nitride edge for boosting photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 297, 120433. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, L.; Fan, X.; Zhou, Y.; Wang, M.; Cheng, R.; Li, M.; Kan, X.; Jin, X.; Liu, Z.; et al. A post-grafting strategy to modify g-C3N4 with aromatic heterocycles for enhanced photocatalytic activity. J. Mater. Chem. A 2016, 4, 13814–13821. [Google Scholar] [CrossRef]
- Dong, H.; Zuo, Y.; Xiao, M.; Zhou, T.; Cheng, S.; Chen, G.; Sun, J.; Yan, M.; Li, C. Limbic inducted and delocalized effects of diazole in carbon nitride skeleton for propelling photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2021, 13, 56273–56284. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Zhang, W.-D. A strategy for integrating transition metal-complex cocatalyst onto g-C3N4 to enable efficient photocatalytic hydrogen evolution. Mol. Catal. 2021, 515, 111856. [Google Scholar] [CrossRef]
- Li, K.; Sun, M.; Zhang, W.-D. Polycyclic aromatic compounds-modified graphitic carbon nitride for efficient visible-light-driven hydrogen evolution. Carbon 2018, 134, 134–144. [Google Scholar] [CrossRef]
- Sun, M.; Guan, H.-X.; Zhang, W.-D.; Yu, Y.-X. Strong organic acid-assistant synthesis of holey graphitic carbon nitride for efficient visible light photocatalytic H2 generation. Int. J. Hydrogen Energy 2019, 44, 23091–23100. [Google Scholar] [CrossRef]
- Su, L.-X.; Huang, Q.-Z.; Lou, Q.; Liu, Z.-Y.; Sun, J.-L.; Zhang, Z.-T.; Qin, S.-R.; Li, X.; Zang, J.-H.; Dong, L.; et al. Effective light scattering and charge separation in nanodiamond@g-C3N4 for enhanced visible-light hydrogen evolution. Carbon 2018, 139, 164–171. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, W.-D. Photocatalytic hydrogen evolution over a nickel complex anchoring to thiophene embedded g-C3N4. J. Colloid Interface Sci. 2021, 596, 75–88. [Google Scholar] [CrossRef]
- Li, K.; Su, F.-Y.; Zhang, W.-D. Modification of g-C3N4 nanosheets by carbon quantum dots for highly efficient photocatalytic generation of hydrogen. Appl. Surf. Sci. 2016, 375, 110–117. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-dimensional porous g-C3N4 for highly efficient photocatalytic overall water splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Müller, J.-O.; Antonietti, M.; Thomas, A. Ionothermal Synthesis of Crystalline, Condensed, Graphitic Carbon Nitride. Chem.–A Eur. J. 2008, 14, 8177–8182. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, Y.; Zhao, Z. Porous defect-modified graphitic carbon nitride via a facile one-step approach with significantly enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B Environ. 2018, 226, 1–9. [Google Scholar] [CrossRef]
- Ye, L.; Wang, D.; Chen, S. Fabrication and enhanced photoelectrochemical performance of MoS2/S-doped g-C3N4 heterojunction film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Tang, S.; Zhang, W.-D.; Yu, Y.-X. Noble metal-free photocatalysts consisting of graphitic carbon nitride, nickel complex, and nickel oxide nanoparticles for efficient hydrogen generation. ACS Appl. Mater. Interfaces 2019, 11, 14986–14996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Yang, C.; Wang, X. Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and separation at a soft photocatalytic interface. Adv. Mater. 2014, 26, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, S.B.; Yang, C.; Lyu, S.H.; Wang, X.C. Modulation of polymeric carbon nitrides through supramolecular preorganization for efficient photocatalytic hydrogen generation. ChemSusChem 2019, 12, 3320–3325. [Google Scholar] [CrossRef]
- Wang, C.; Fu, M.; Cao, J.; Wu, X.; Hu, X.; Dong, F. BaWO4/g-C3N4 heterostructure with excellent bifunctional photocatalytic performance. Chem. Eng. J. 2019, 385, 123833. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Qi, R.; Cao, C.; Liu, X.; Zheng, Y.; Song, W. Enhanced electron separation on in-plane benzene-ring doped g-C3N4 nanosheets for visible light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 244, 459–464. [Google Scholar] [CrossRef]
- Chen, J.; Dong, C.-L.; Zhao, D.; Huang, Y.-C.; Wang, X.; Samad, L.; Dang, L.; Shearer, M.; Shen, S.; Guo, L. Molecular design of polymer heterojunctions for efficient solar-hydrogen conversion. Adv. Mater. 2017, 29, 1606198. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 54, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Lin, L.; Gim, S.; Lee, S.; Kim, H.; Wang, X.; Choi, W. Polymeric carbon nitride with localized aluminum coordination sites as a durable and efficient photocatalyst for visible light utilization. ACS Catal. 2018, 8, 4241–4256. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Fu, X.; Wang, X. Construction of conjugated carbon nitride nanoarchitectures in solution at low temperatures for photoredox catalysis. Angew. Chem. Int. Ed. 2012, 51, 11814–11818. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, J.; Zhang, M.; Antonietti, M.; Fu, X.; Wang, X. Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles. Nat. Commun. 2012, 3, 1139. [Google Scholar] [CrossRef]
- Chan-Chan, L.H.; González-García, G.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Hernández-Sánchez, F.; Marcos-Fernandez, A.; Cauich-Rodríguez, J.V. Characterization of model compounds and poly(amide-urea) urethanes based on amino acids by FTIR, NMR and other analytical techniques. Eur. Polym. J. 2017, 92, 27–39. [Google Scholar] [CrossRef]
- Cao, S.-W.; Yuan, Y.-P.; Barber, J.; Loo, S.C.J.; Xue, C. Noble-metal-free g-C3N4/Ni(dmgH)2 composite for efficient photocatalytic hydrogen evolution under visible light irradiation. Appl. Surf. Sci. 2014, 319, 344–349. [Google Scholar] [CrossRef]
- Li, Q.; Ren, C.; Qiu, C.; He, T.; Zhang, Q.; Ling, X.; Xu, Y.; Su, C. Promoting near-infrared photocatalytic activity of carbon-doped carbon nitride via solid alkali activation. Chin. Chem. Lett. 2021, 32, 3463–3468. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.-C.; Kang, X.; Wang, L.; Liu, G.; Cheng, H.-M. Selective breaking of hydrogen bonds of layered carbon nitride for visible light photocatalysis. Adv. Mater. 2016, 28, 6471–6477. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, W.; Gao, W.; Li, P.; Wang, X.; Wu, S.; Song, W.; Ding, K. Aromatic ring substituted g-C3N4 for enhanced photocatalytic hydrogen evolution. J. Mater. Chem. A 2017, 5, 17199–17203. [Google Scholar] [CrossRef]
- Moon, H.S.; Hsiao, K.; Wu, M.; Yun, Y.; Hsu, Y.; Yong, K. Spatial Separation of Cocatalysts on Z-Scheme Organic/Inorganic Heterostructure Hollow Spheres for Enhanced Photocatalytic H 2 Evolution and In-Depth Analysis of the Charge-Transfer Mechanism. Adv. Mater. 2022, 2200172. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Yu, J.; Zhang, Y. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
- Han, Q.; Hu, C.; Zhao, F.; Zhang, Z.; Chen, N.; Qu, L. One-step preparation of iodine-doped graphitic carbon nitride nanosheets as efficient photocatalysts for visible light water splitting. J. Mater. Chem. A 2015, 3, 4612–4619. [Google Scholar] [CrossRef]
- Guo, Y.; Chu, S.; Yan, S.; Wang, Y.; Zou, Z. Developing a polymeric semiconductor photocatalyst with visible light response. Chem. Commun. 2010, 46, 7325–7327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lu, G.; Liu, J.; Wu, D.; Yan, Z.; Wang, Y. Incorporation of π-conjugated molecules as electron donors in g-C3N4 enhances photocatalytic H2-production. Renew. Energy 2020, 164, 531–540. [Google Scholar] [CrossRef]













| Sample | Atomic Ratio (%) | C/N | |||
|---|---|---|---|---|---|
| C | N | O | S | ||
| CN | 46.74 | 48.66 | 4.60 | — | 0.96 |
| TA-CN-2 | 47.07 | 47.93 | 4.57 | 0.43 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Xu, Y.-S.; Zhang, W.-D. Embedding Thiophene-Amide into g-C3N4 Skeleton with Induction and Delocalization Effects for High Photocatalytic H2 Evolution. Catalysts 2022, 12, 1043. https://doi.org/10.3390/catal12091043
Tang S, Xu Y-S, Zhang W-D. Embedding Thiophene-Amide into g-C3N4 Skeleton with Induction and Delocalization Effects for High Photocatalytic H2 Evolution. Catalysts. 2022; 12(9):1043. https://doi.org/10.3390/catal12091043
Chicago/Turabian StyleTang, Shuang, Yang-Sen Xu, and Wei-De Zhang. 2022. "Embedding Thiophene-Amide into g-C3N4 Skeleton with Induction and Delocalization Effects for High Photocatalytic H2 Evolution" Catalysts 12, no. 9: 1043. https://doi.org/10.3390/catal12091043
APA StyleTang, S., Xu, Y.-S., & Zhang, W.-D. (2022). Embedding Thiophene-Amide into g-C3N4 Skeleton with Induction and Delocalization Effects for High Photocatalytic H2 Evolution. Catalysts, 12(9), 1043. https://doi.org/10.3390/catal12091043