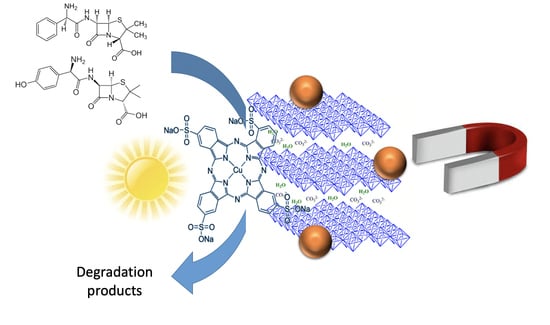
Use of Photocatalytically Active Supramolecular Organic–Inorganic Magnetic Composites as Efficient Route to Remove β-Lactam Antibiotics from Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. X-ray Diffraction (XRD)
2.1.2. Diffuse Reflectance UV-Vis Spectroscopy (DR-UV-Vis)
2.1.3. Diffuse-Reflectance Infrared Spectroscopy (DRIFT)
2.1.4. Textural Properties
2.2. Degradation of Antibiotics
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.2.1. Specific Surface Area (BET) and Pore-Size Measurements
3.2.2. X-ray Diffraction (XRD)
- k = 0.9 (shape factor)
- λ = wavelength of the X-ray radiation source (for Cu = 1.54 Å)
- FWHM = full width at half height (in radians)
- θ = maximum position (in radians)
3.2.3. Diffuse-Reflectance UV-Vis Spectroscopy (DR-UV-Vis)
3.2.4. Diffuse-Reflectance Infrared Spectroscopy (DRIFT)
3.3. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ji, S.; Xi, B. Feasibility study of treatment of amoxillin wastewater with a combination of extraction, Fenton oxidation and reverse osmosis. Desalination 2006, 196, 32–42. [Google Scholar] [CrossRef]
- Surenjan, A.; Pradeep, T.; Philip, L. Application and performance evaluation of a cost-effective vis- LED based fluidized bed reactor for the treatment of emerging contaminants. Chemosphere 2019, 228, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2022, 430, 132806. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, S.; Shi, M.; Hong, X.; Wang, D.; Wang, F.; Xia, M.; Chen, Q. Enhanced photocatalytic degradation performance of BiVO4/BiOBr through combining Fermi level alteration and oxygen defect engineering. Chem. Eng. J. 2022, 449, 137757. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Bobo, M.V.; Kuchta, J.J.I.; Vannucci, A.K. Recent advancements in the development of molecular organic photocatalysts. Org. Biomol. Chem. 2021, 19, 4816–4834. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075−10166. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Calvete, M.J.F. Phthalocyanines: An Old Dog Can Still Have New (Photo)Tricks! Molecules 2021, 26, 2823. [Google Scholar] [CrossRef]
- Vallejo Lozada, W.A.; Diaz-Uribe, C.; Quiñones, C.; Lerma, M.; Fajardo, C.; Navarro, K. Phthalocyanines: Alternative Sensitizers of TiO2 to be Used in Photocatalysis. In Phthalocyanines and Some Current Applications; Yilmaz, Y., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Ion, R. Porphyrins and Phthalocyanines: Photosensitizers and Photocatalysts. In Phthalocyanines and Some Current Applications; Yilmaz, Y., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Zeolite-based photocatalysts. Chem. Commun. 2004, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, K.T.; Willner, I.; Bossmann, S.; Braun, A. Iron(III) phthalocyanine-modified titanium dioxide: A novel photocatalyst for the enhanced photodegradation of organic pollutants. J. Phys. Chem. B 1998, 102, 9397–9403. [Google Scholar] [CrossRef]
- Zsigmond, A.; Notheisz, F.; Bäckvall, J.-E. Rate enhancement of oxidation reactions by the encapsulation of metal phthalocyanine complexes. Catal. Lett. 2000, 65, 135–139. [Google Scholar] [CrossRef]
- Perez-Bemal, E.; Ruano-Casero, R.; Pinnavaia, T.J. Catalytic autoxidation of 1-decanethiol by cobalt(II) phthalocyaninetetrasulfonate intercalated in a layered double hydroxide. Catal. Lett. 1991, 11, 55. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. Mater. Chem. A 2016, 4, 10744. [Google Scholar] [CrossRef]
- Gao, L.-G.; Gao, Y.-Y.; Song, X.-L.; Ma, X.-R. A novel La3+-Zn2+-Al3+-MoO42− layered double hydroxides photocatalyst for the decomposition of dibenzothiophene in diesel oil. Pet. Sci. Technol. 2018, 36, 850–855. [Google Scholar] [CrossRef]
- Barbosa, C.A.S.; Ferrira, A.M.D.C.; Constantino, V.R.L.; Coelho, A.C.V. Preparation and Characterization of Cu(II) Phthalocyanine Tetrasulfonate Intercalated and Supported on Layered Double Hydroxides. J. Incl. Phenom. Macro. Chem. 2002, 42, 15. [Google Scholar] [CrossRef]
- Barbosa, C.A.S.; Dias, P.M.; Ferrira, A.M.C.; Constantino, V.R.L. Mg–Al hydrotalcite-like compounds containing iron-phthalocyanine complex: Effect of aluminum substitution on the complex adsorption features and catalytic activity. Appl. Clay. Sci. 2005, 28, 147. [Google Scholar] [CrossRef]
- Barbosa, C.A.S.; Ferrira, A.M.D.C.; Constantino, V.R.L. Synthesis and Characterization of Magnesium-Aluminum Layered Double Hydroxides Containing (Tetrasulfonated porphyrin)cobalt. Eur. J. Inorg. Chem. 2005, 1577. [Google Scholar] [CrossRef]
- Kanan, S.; Awate, S.V.; Agashe, M.S. Incorporation of anionic copper phthalocyanine complexes into the intergallery of Mg-Al layered double hydroxides. Stud. Surf. Sci. Catal. 1998, 113, 927. [Google Scholar] [CrossRef]
- Parida, K.M.; Baliarsingh, N.; Sairam Patra, B.; Das, J. Copperphthalocyanine immobilized Zn/Al LDH as photocatalyst under solar radiation for decolorization of methylene blue. J. Mol. Catal. A Chem. 2007, 267, 202–208. [Google Scholar] [CrossRef]
- Maretti, L.; Carbonell, E.; Alvaro, M.; Scaiano, J.C.; Garcia, H. Laser flash photolysis of dioxo iron phthalocyanine intercalated in hydrotalcite and its use as a photocatalyst. J. Photochem. Photobio. A Chem. 2009, 205, 19–22. [Google Scholar] [CrossRef]
- Abu-Reziq, R.; Alper, H.; Wang, D.; Post, M.L. Metal Supported on Dendronized Magnetic Nanoparticles: Highly Selective Hydroformylation Catalysts. J. Am. Chem. Soc. 2006, 128, 5279–5282. [Google Scholar] [CrossRef]
- Karaoğlu, E.; Baykal, A.; Erdemi, H.; Alpsoy, L.; Sozeri, H. Synthesis and characterization of dl-thioctic acid (DLTA)–Fe3O4 nanocomposite. J. Alloys Compd. 2011, 509, 9218–9225. [Google Scholar] [CrossRef]
- Naeimi, H.; Nazifi, Z.S. A highly efficient nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups as a solid acid catalyst for synthesis of 1,8-dioxo-octahydroxanthene derivatives. J. Nanopart. Res. 2013, 15, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126198. [Google Scholar] [CrossRef]
- Szabó, L.; Tóth, T.; Engelhardt, T.; Rácz, G.; Mohácsi-Farkas, S.; Takács, E.; Wojnárovits, L. Change in hydrophilicity of penicillinsduring advanced oxidation by radiolytically generated OH compromises the elimination of selective pressure on bacterial strains. Sci. Total Environ. 2016, 551–552, 393–403. [Google Scholar] [CrossRef]
- Andreozzi, R.; Canterino, M.; Marotta, R.; Paxeus, N. Antibiotic removal from wastewaters: The ozonation of amoxicillin. J. Hazard. Mater. 2005, 122, 243–250. [Google Scholar] [CrossRef]
- Rozas, O.; Contreras, D.; Mondaca, M.A.; Pérez-Moya, M.; Mansilla, H.D. Experimental design of Fenton and photo-Fenton reactions for the treatment of ampicillin solutions. J. Haz. Mat. 2010, 177, 1025–1030. [Google Scholar] [CrossRef]
- Belhacova, L.; Bibova, H.; Marikova, T.; Kuchar, M.; Zouzelka, R.; Rathousky, J. Removal of Ampicillin by Heterogeneous Photocatalysis: Combined Experimental and DFT Study. Nanomaterials 2021, 11, 1992. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, miner-alization and antibiotic inactivation of AMX by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Klauson, D.; Babkina, J.; Stepanova, K.; Krichevskaya, M.; Preis, S. Aqueous photocatalytic oxidation of amoxicillin. Catal. Today 2010, 151, 39–45. [Google Scholar] [CrossRef]
- Shaykhi, Z.M.; Zinatizadeh, A.A.L. Statistical modeling of photocatalytic degradation of synthetic AMX wastewater in an immobilized TiO2 photocatalytic reactor using response surface methodology. J. Taiwan Inst. Chem. Eng. 2014, 45, 1717–1726. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibioticresistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Pouretedala, H.R.; Hasanali, M.A. Photocatalytic degradation of some b-lactam antibiotics in aqueous suspension of ZnS nanoparticles. Desalination Water Treat. 2013, 51, 2617–2623. [Google Scholar] [CrossRef]
- Nosrati, R.; Olad, A.; Maramifar, R. Degradation of ampicillin antibiotic in aqueous solution by ZnO/polyaniline nanocomposite as photocatalyst under sunlight irradiation. Environ. Sci. Pollut. Res. 2012, 19, 2291–2299. [Google Scholar] [CrossRef]
- Jassal, P.S.; Khajuria, R.; Sharma, R.; Debnath, P.; Verma, S.; Johnson, A.; Kumar, S. Photocatalytic degradation of ampicillin using silver nanoparticles biosynthesised by Pleurotus ostreatus. BioTechnologia 2020, 101, 5–14. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Mihăilă, S.-D.; Cojocaru, B.; Tudorache, M.; Parvulescu, V.I.; Pavel, O.D.; Oikonomopoulos, S.; Jacobsen, E.E. An advanced approach for MgZnAl-LDH catalysts synthesis used in Claisen-Schmidt condensation. Catalysts 2022, 12, 759. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Zăvoianu, R.; Bucur, I.C.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechano-chemical versus co-precipitation for the preparation of Y-modified LDHs for cyclohexene oxidation and Claisen-Schmidt condensations. Appl. Catal. A Gen. 2020, 605, 117797. [Google Scholar] [CrossRef]
- Prévot, V.; Casala, B.; Ruiz-Hitzky, E. Intracrystalline alkylation of benzoate ions into layered double hydroxides. J. Mater. Chem. 2001, 11, 554–560. [Google Scholar] [CrossRef]
- Mastalir, Á.; Király, Z. Pd nanoparticles in hydrotalcite: Mild and highly selective catalysts for alkyne semihydrogenation. J. Catal. 2003, 220, 372–381. [Google Scholar] [CrossRef]
- Carja, G.; Delahay, G. Mesoporous mixed oxides derived from pillared oxovanadates layered double hydroxides as new catalysts for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2004, 47. [Google Scholar] [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-Like Compounds and Their Structures and Physico-Chemical Properties—I: The Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B.; Tajally, M.; Asghari, A. Optical properties of MgO and Mg(OH)2 nanostructures synthesized by a chemical precipitation method using impure brine. J. Alloys Compd. 2017, 711, 521–529. [Google Scholar] [CrossRef]
- Sakamoto, K.; Ohno-Okumura, E. Syntheses and Functional Properties of Phthalocyanines. Materials 2009, 2, 1127–1179. [Google Scholar] [CrossRef]
- Zhang, C.; Tong, S.W.; Jiang, C.; Kang, E.T.; Chan, D.S.H.; Zhu, C. Simple tandem organic photovoltaic cells for improved energy conversion efficiency. Appl. Phys. Lett. 2008, 92, 68. [Google Scholar] [CrossRef]
- Rajendran, K.; Balakrishnan, G.S.; Kalirajan, J. Synthesis of Magnetite Nanoparticles for Arsenic Removal from Ground Water Pond. Int. J. PharmTech Res. 2015, 8, 670–677. [Google Scholar]
- Pavel, O.D.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechanochemical versus co-precipitated synthesized lanthanum-doped layered materials for olefin oxidation. Appl. Catal. A Gen. 2017, 542, 10–20. [Google Scholar] [CrossRef]
- Huang, F.; Tian, S.; Qi, Y.; Li, E.; Zhou, L.; Qiu, Y. Synthesis of FePcS-PMA-LDH Cointercalation Composite with Enhanced Visible Light Photo-Fenton Catalytic Activity for BPA Degradation at Circumneutral pH. Materials 2020, 13, 1951. [Google Scholar] [CrossRef] [Green Version]
- Hur, T.-B.; Phuoc, T.X.; Chyu, M.K. New approach to the synthesis of layered double hydroxides and associated ultrathin nanosheets in de-ionized water by laser ablation. J. Appl. Phys. 2010, 108, 114312. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, L.; Monduzzi, M.; Salis, A. Adsorption and release of ampicillin antibiotic from ordered mesoporous silica. J. Colloid Interface Sci. 2017, 497, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Elhaci, A.; Labed, F.; Khenifi, A.; Bouberka, Z.; Kameche, M.; Benabbou, K. MgAl-Layered double hydroxide for amoxicillin removal from aqueous media. J. Environ. Anal. Chem. 2020, 101, 2876–2898. [Google Scholar] [CrossRef]
- Dogan, S.; Kidak, R. A Plug flow reactor model for UV-based oxidation of amoxicillin. Desalin. Water Treat. 2016, 57, 13586–13599. [Google Scholar] [CrossRef]









| Sample | Precursor | Conditions |
|---|---|---|
| P1 | FeCl2 + Fe(NO3)3 | LDH:Fe3O4 = 1:3 (10 min ageing) |
| P2 | FeCl2 + Fe(NO3)3 | LDH:Fe3O4 = 1:3 (30 min ageing) |
| P3 | FeCl2 + Fe(NO3)3 | LDH:Fe3O4 = 3:1 (60 min ageing) |
| P4 | FeCl2 + Fe(NO3)3 | LDH:Fe3O4 = 3:2 (60 min ageing) |
| P5 | FeCl2 + Fe(NO3)3 | LDH:Fe3O4 = 1:1 (60 min ageing) |
| P6 | FeCl2 + Fe(NO3)3 | LDH:Fe3O4 = 1:2 (60 min ageing) |
| P7 | FeSO4 + FeCl3 (2:1 mol) | LDH:Fe3O4 = 3:1 (60 min ageing) |
| P8 | FeSO4 + FeCl3 (1:2 mol) | LDH:Fe3O4 = 3:1 (60 min ageing) |
| Sample | a (Å) | c (Å) | IFS (Å) 1 | I003/I006 | I003/I110 | D(Å) 2 |
|---|---|---|---|---|---|---|
| LDH–MgZnAl | 3.0717 | 22.8689 | 2.82 | 2.96 | 5.54 | 132 |
| P1 | 3.0462 | 22.5822 | 2.73 | 1.00 | 0.67 | 111 |
| P2 | 3.0557 | 22.6262 | 2.74 | 1.08 | 0.68 | 115 |
| P3 | 3.0616 | 22.9058 | 2.84 | 2.21 | 4.89 | 96 |
| P4 | 3.0539 | 22.8707 | 2.82 | 2.21 | 6.73 | 105 |
| P5 | 3.0644 | 23.1147 | 2.90 | 1.95 | 4.56 | 79 |
| P6 | 3.0663 | 23.5299 | 3.04 | 3.00 | 1.29 | 200 |
| P7 | 3.0612 | 22.9415 | 2.85 | 2.17 | 3.98 | 97 |
| P8 | 3.0589 | 22.8956 | 2.83 | 2.20 | 4.04 | 95 |
| Sample | Ss (BET) (m2∙g−1) | Pore Volume (cm3∙g−1) | Average Pore Size (Å) |
|---|---|---|---|
| LDH-MgZnAl | 69 | 0.387 | 222 |
| Rehydrated LDH-MgZnAl | 25 | 0.186 | 238 |
| P1 | 129 | 0.277 | 72 |
| P2 | 116 | 0.258 | 73 |
| P3 | 137 | 0.858 | 34, 317 |
| P4 | 111 | 0.392 | 34, 90, 231 |
| P5 | 163 | 0.336 | 37, 63, 182 |
| P6 | 148 | 0.230 | 36, 45 |
| P7 | 124 | 0.628 | ~200 |
| P8 | 124 | 0.498 | ~200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, S.G.; Pavel, O.D.; Guzo, N.; Tudorache, M.; Coman, S.M.; Parvulescu, V.I.; Cojocaru, B.; Jacobsen, E.E. Use of Photocatalytically Active Supramolecular Organic–Inorganic Magnetic Composites as Efficient Route to Remove β-Lactam Antibiotics from Water. Catalysts 2022, 12, 1044. https://doi.org/10.3390/catal12091044
Ion SG, Pavel OD, Guzo N, Tudorache M, Coman SM, Parvulescu VI, Cojocaru B, Jacobsen EE. Use of Photocatalytically Active Supramolecular Organic–Inorganic Magnetic Composites as Efficient Route to Remove β-Lactam Antibiotics from Water. Catalysts. 2022; 12(9):1044. https://doi.org/10.3390/catal12091044
Chicago/Turabian StyleIon, Sabina G., Octavian D. Pavel, Nicolae Guzo, Madalina Tudorache, Simona M. Coman, Vasile I. Parvulescu, Bogdan Cojocaru, and Elisabeth E. Jacobsen. 2022. "Use of Photocatalytically Active Supramolecular Organic–Inorganic Magnetic Composites as Efficient Route to Remove β-Lactam Antibiotics from Water" Catalysts 12, no. 9: 1044. https://doi.org/10.3390/catal12091044
APA StyleIon, S. G., Pavel, O. D., Guzo, N., Tudorache, M., Coman, S. M., Parvulescu, V. I., Cojocaru, B., & Jacobsen, E. E. (2022). Use of Photocatalytically Active Supramolecular Organic–Inorganic Magnetic Composites as Efficient Route to Remove β-Lactam Antibiotics from Water. Catalysts, 12(9), 1044. https://doi.org/10.3390/catal12091044