A Review of Persulfate Activation by Magnetic Catalysts to Degrade Organic Contaminants: Mechanisms and Applications
Abstract
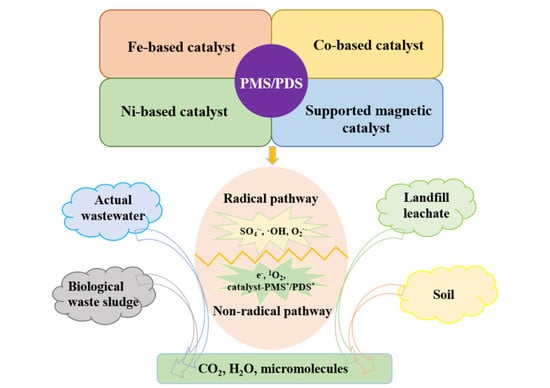
:1. Introduction
2. Magnetic Catalysts for Persulfate Activation
2.1. Zero-Valent Iron (ZVI, Fe0)
2.2. Iron Oxide
2.3. Nickel-Cobalt Bimetallic Catalyst
2.4. Supported Magnetic Catalyst
3. Mechanism for Persulfate Activation by Magnetic Catalysts
3.1. Identification of Reactive Oxygen Species
3.2. Radical Pathway and Non-Radical Pathway
3.2.1. Radical Pathway
3.2.2. Non-Radical Pathway
3.2.3. The Synergetic Radical Pathway and Non-Radical Pathway
4. The Application of the Persulfate/Magnetic Catalyst System
4.1. Actual Wastewater
4.2. Landfill Leachate
4.3. Biological Waste Sludge
4.4. Soil
5. Challenges and Perspectives
- (1)
- According to the magnetic species, magnetic catalysts can be roughly divided into iron-based catalyst, cobalt-based catalyst, nickel-based catalyst, and supported magnetic catalyst, and the most common is the magnetic carbon composite catalyst. However, the magnetic catalyst/persulfate system is still in the development stage and is rarely used in actual wastewater. The actual wastewater composition is complex, and in future research, we can design catalyst materials with better performance or optimize existing materials to cope with the complex composition and environment of the actual wastewater.
- (2)
- The reaction mechanism of magnetic catalyst activation of persulfate includes the radical pathway and the non-radical pathway, which is related to the nature of the catalyst. However, some studies are not comprehensive enough for the radicals trapping experiments and EPR/ESR experiments involved in the reaction system, which may lead to a lack of comprehensive understanding of the reaction mechanism. In future research, we can combine representational means and experimental means to carry out more in-depth and comprehensive research and summary.
- (3)
- A variety of magnetic catalysts can be used to treat refractory organic pollutants in the aqueous phase, including phenols, antibiotics, dyes, chlorinated organic pollutants, etc., but these are in the research stage of laboratory model wastewater, and there are few cases of actual wastewater treatment. In future research, the magnetic catalyst and magnetic catalyst/persulfate technology can be optimized and perfected. It is necessary to consider the switch from the laboratory scale to the middle scale, and apply the magnetic catalyst-activated persulfate system with high efficiency and low energy consumption, and make recovery of the treatment of refractory organic pollutants easy in the actual environment as soon as possible.
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Gosset, A.; Ferro, Y.; Durrieu, C. Methods for evaluating the pollution impact of urban wet weather discharges on biocenosis: A review. Water Res. 2016, 89, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Huang, D.; Sun, X.; Liu, Y.; Ji, H.; Liu, W.; Wang, C.-C.; Ma, W.; Cai, Z. A carbon-rich g-C3N4 with promoted charge separation for highly efficient photocatalytic degradation of amoxicillin. Chin. Chem. Lett. 2021, 32, 2787–2791. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wang, P.; Yin, L.; Tian, Y.; Li, J. Bifunctional copper modified graphitic carbon nitride catalysts for efficient tetracycline removal: Synergy of adsorption and photocatalytic degradation. Chin. Chem. Lett. 2020, 31, 2789–2794. [Google Scholar] [CrossRef]
- Ozyildiz, G.; Olmez-Hanci, T.; Arslan-Alaton, I. Effect of nano-scale, reduced graphene oxide on the degradation of bisphenol A in real tertiary treated wastewater with the persulfate/UV-C process. Appl. Catal. B-Environ. 2019, 254, 135–144. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Ji, F.; Lai, B. Heterogeneous catalytic oxidation for the degradation of p-nitrophenol in aqueous solution by persulfate activated with CuFe2O4 magnetic nano-particles. Chem. Eng. J. 2017, 324, 63–73. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Qu, J.H.; Tian, X.; Zhang, X.B.; Yao, J.Y.; Xue, J.Q.; Li, K.G.; Zhang, B.; Wang, L.; Zhang, Y. Free radicals-triggered reductive and oxidative degradation of highly chlorinated compounds via regulation of heat-activated persulfate by low-molecular-weight organic acids. Appl. Catal. B-Environ. 2022, 310, 121359. [Google Scholar] [CrossRef]
- Qu, J.H.; Xu, Y.; Zhang, X.B.; Sun, M.Z.; Tao, Y.; Zhang, X.M.; Zhang, G.S.; Ge, C.J.; Zhang, Y. Ball milling-assisted preparation of N-doped biochar loaded with ferrous sulfide as persulfate activator for phenol degradation: Multiple active sites-triggered radical/non-radical mechanism. Appl. Catal. B-Environ. 2022, 316, 121639. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, P.; Li, J.; Wang, Q.; Xu, P.; You, S.; Zheng, Q.; Zhang, G. Photocatalytic PVDF ultrafiltration membrane blended with visible-light responsive Fe(III)-TiO2 catalyst: Degradation kinetics, catalytic performance and reusability. Chem. Eng. J. 2021, 417, 129340. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, S. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A critical review of advanced oxidation processes for emerging trace organic contaminant degradation: Mechanisms, factors, degradation products, and effluent toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Bennajah, M.; Elkacmi, R. Advanced oxidation technologies for the treatment and detoxification of olive mill wastewater: A general review. J. Water Reuse Desalin. 2019, 9, 463–505. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Wang, D.; Xu, Q.; Yang, Q.; Zeng, G.; Li, X.; Liu, Y.; Gong, J.; Ye, J.; et al. Mechanisms of peroxymonosulfate pretreatment enhancing production of short-chain fatty acids from waste activated sludge. Water Res. 2019, 148, 239–249. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B-Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Neyens, E. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J. Hazard. Mater. 2004, 106, 83–92. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.; Ifthikar, J.; Shi, L.; Du, Y.; Zhu, J.; Xi, S.; Chen, Z.; Chen, Z. Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process. Chemosphere 2017, 185, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Gu, L.; Wu, D.; Liu, Z. Mackinawite (FeS) activation of persulfate for the degradation of p-chloroaniline: Surface reaction mechanism and sulfur-mediated cycling of iron species. Chem. Eng. J. 2018, 333, 657–664. [Google Scholar] [CrossRef]
- Zhu, L.; Ai, Z.; Ho, W.; Zhang, L. Core–shell Fe–Fe2O3 nanostructures as effective persulfate activator for degradation of methyl orange. Sep. Purif. Technol. 2013, 108, 159–165. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate Radical and Its Application in Decontamination Technologies. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1756–1800. [Google Scholar] [CrossRef]
- Karim, A.V.; Jiao, Y.; Zhou, M.; Nidheesh, P.V. Iron-based persulfate activation process for environmental decontamination in water and soil. Chemosphere 2021, 265, 129057. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yang, S.; Wacławek, S.; Fang, G.; Xiao, R.; Dionysiou, D.D. Limitations and prospects of sulfate-radical based advanced oxidation processes. J. Environ. Chem. Eng. 2020, 8, 103849. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B-Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Li, C.-X.; Chen, C.-B.; Lu, J.-Y.; Cui, S.; Li, J.; Liu, H.-Q.; Li, W.-W.; Zhang, F. Metal organic framework-derived CoMn2O4 catalyst for heterogeneous activation of peroxymonosulfate and sulfanilamide degradation. Chem. Eng. J. 2018, 337, 101–109. [Google Scholar] [CrossRef]
- Wang, F.; Wu, C.; Li, Q. Treatment of refractory organics in strongly alkaline dinitrodiazophenol wastewater with microwave irradiation-activated persulfate. Chemosphere 2020, 254, 126773. [Google Scholar] [CrossRef]
- Mahdi-Ahmed, M.; Chiron, S. Ciprofloxacin oxidation by UV-C activated peroxymonosulfate in wastewater. J. Hazard. Mater. 2014, 265, 41–46. [Google Scholar] [CrossRef]
- Fedorov, K.; Plata-Gryl, M.; Khan, J.A.; Boczkaj, G. Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. J. Hazard. Mater. 2020, 397, 122804. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of functionalized magnetic biochar in environmental remediation: A review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef]
- Yin, F.; Wang, C.; Lin, K.-Y.A.; Tong, S. Persulfate activation for efficient degradation of norfloxacin by a rGO-Fe3O4 composite. J. Taiwan Inst. Chem. Eng. 2019, 102, 163–169. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Liu, G.; Zheng, Q.; Zhang, G. Catalytic degradation of p-nitrophenol by magnetically recoverable Fe3O4 as a persulfate activator under microwave irradiation. Chemosphere 2020, 240, 124977. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, S.-S.; Geng, Y.; Zhen, J.; Zhan, J.; Cao, C.; Ni, S.-Q. Synergistic catalysis by Fe3O4-biochar/peroxymonosulfate system for the removal of bisphenol a. Sep. Purif. Technol. 2021, 276, 119351. [Google Scholar] [CrossRef]
- Rodriguez, S.; Santos, A.; Romero, A. Oxidation of priority and emerging pollutants with persulfate activated by iron: Effect of iron valence and particle size. Chem. Eng. J. 2017, 318, 197–205. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Blaney, L.; Peng, G.; Chen, P.; Cui, Y.; Deng, S.; Wang, Y.; Huang, J.; Yu, G. Degradation of sulfamethazine by persulfate activated with organo-montmorillonite supported nano-zero valent iron. Chem. Eng. J. 2019, 361, 99–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Pan, Y.; Xu, L.; Zhou, M. Pre-magnetized Fe0 activated persulphate for the degradation of nitrobenzene in groundwater. Sep. Purif. Technol. 2019, 212, 555–562. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Su, L.; Kobayashi, T.; Kumar, G.; Zhou, T.; Xu, K.; Li, Y.Y.; Zhu, X.; Zhao, Y. Unraveling the catalyzing behaviors of different iron species (Fe(2+) vs. Fe(0)) in activating persulfate-based oxidation process with implications to waste activated sludge dewaterability. Water Res. 2018, 134, 101–114. [Google Scholar] [CrossRef]
- Hou, K.; Pi, Z.; Yao, F.; Wu, B.; He, L.; Li, X.; Wang, D.; Dong, H.; Yang, Q. A critical review on the mechanisms of persulfate activation by iron-based materials: Clarifying some ambiguity and controversies. Chem. Eng. J. 2021, 407, 127078. [Google Scholar] [CrossRef]
- Palharim, P.H.; Graca, C.A.L.; Teixeira, A. Comparison between UVA- and zero-valent iron-activated persulfate processes for degrading propylparaben. Environ. Sci. Pollut. Res. Int. 2020, 27, 22214–22224. [Google Scholar] [CrossRef] [PubMed]
- Hayat, W.; Zhang, Y.; Hussain, I.; Du, X.; Du, M.; Yao, C.; Huang, S.; Si, F. Efficient degradation of imidacloprid in water through iron activated sodium persulfate. Chem. Eng. J. 2019, 370, 1169–1180. [Google Scholar] [CrossRef]
- El Fakir, A.A.; Anfar, Z.; Amedlous, A.; Amjlef, A.; Farsad, S.; Jada, A.; El Alem, N. Synergistic effect for efficient catalytic persulfate activation in conducting polymers-hematite sand composites: Enhancement of chemical stability. Appl. Catal. A-Gen. 2021, 623, 118246. [Google Scholar] [CrossRef]
- Yang, M.; Ren, X.; Hu, L.; Guo, W.; Zhan, J. Facet-controlled activation of persulfate by goethite for tetracycline degradation in aqueous solution. Chem. Eng. J. 2021, 412, 128628. [Google Scholar] [CrossRef]
- Lv, J.; Gong, L.; Chen, X.; Liu, B.; Li, Y.; Jiang, J.; Zhou, J. Enhancements of short-chain fatty acids production via anaerobic fermentation of waste activated sludge by the combined use of persulfate and micron-sized magnetite. Bioresour. Technol. 2021, 342, 126051. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ren, J.; Liu, J.; Rong, L.; Wang, H.; Xiao, Y.; Sun, F.; Mei, R.; Chen, C.; Su, X. Pyrite-activated persulfate oxidation and biological denitrification for effluent of biological landfill leachate treatment system. J. Environ. Manag. 2022, 304, 114290. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Sun, C.; Sun, J.Q.; Zhou, R. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep. Purif. Technol. 2015, 142, 182–188. [Google Scholar] [CrossRef]
- Yan, J.; Lei, M.; Zhu, L.; Anjum, M.N.; Zou, J.; Tang, H. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate. J. Hazard. Mater. 2011, 186, 1398–1404. [Google Scholar] [CrossRef]
- Hussain, I.; Zhang, Y.; Li, M.; Huang, S.; Hayat, W.; He, L.; Du, X.; Liu, G.; Du, M. Heterogeneously degradation of aniline in aqueous solution using persulfate catalyzed by magnetic BiFeO3 nanoparticles. Catal. Today 2018, 310, 130–140. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef]
- Tian, X.; Tian, C.; Nie, Y.; Dai, C.; Yang, C.; Tian, N.; Zhou, Z.; Li, Y.; Wang, Y. Controlled synthesis of dandelion-like NiCo2O4 microspheres and their catalytic performance for peroxymonosulfate activation in humic acid degradation. Chem. Eng. J. 2018, 331, 144–151. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Zou, D.; Yuan, X.; Xiao, Z.; Deng, Y.; Zhou, Y.; Jiang, L.; Qin, P. Enhanced heterogeneous activation of persulfate by NixCo3–xO4 for oxidative degradation of tetracycline and bisphenol A. J. Environ. Chem. Eng. 2020, 8, 104451. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Dong, S.; Wang, P. Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation. Sci. Total Environ. 2019, 647, 352–361. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Dong, Y.D.; Zhang, L.Q.; Zhou, P.; Liu, Y.; Lin, H.; Zhong, G.J.; Yao, G.; Li, Z.M.; Lai, B. Natural cellulose supported carbon nanotubes and Fe3O4 NPs as the efficient peroxydisulfate activator for the removal of bisphenol A: An enhanced non-radical oxidation process. J. Hazard. Mater. 2022, 423, 127054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, B.T.; Teng, Y.; Zhao, J.; Sun, X. Heterogeneous activation of persulfate by carbon nanofiber supported Fe3O4@carbon composites for efficient ibuprofen degradation. J. Hazard. Mater. 2021, 401, 123428. [Google Scholar] [CrossRef]
- Xu, P.; Wang, P.; Wang, Q.; Wei, R.; Li, Y.; Xin, Y.; Zheng, T.; Hu, L.; Wang, X.; Zhang, G. Facile synthesis of Ag2O/ZnO/rGO heterojunction with enhanced photocatalytic activity under simulated solar light: Kinetics and mechanism. J. Hazard. Mater. 2021, 403, 124011. [Google Scholar] [CrossRef]
- Peng, Y.; Azeem, M.; Li, R.; Xing, L.; Li, Y.; Zhang, Y.; Guo, Z.; Wang, Q.; Ngo, H.H.; Qu, G.; et al. Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: Application for As(III) and As(V) polluted water purification. J. Hazard. Mater. 2022, 423, 127081. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Huang, Z.; Bao, H.; Yao, Y.; Lu, W.; Chen, W. Novel green activation processes and mechanism of peroxymonosulfate based on supported cobalt phthalocyanine catalyst. Appl. Catal. B-Environ. 2014, 154–155, 36–43. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Wang, P. Enhanced degradation of Bisphenol A (BPA) by peroxymonosulfate with Co3O4-Bi2O3 catalyst activation: Effects of pH, inorganic anions, and water matrix. Chem. Eng. J. 2018, 338, 300–310. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Enhanced peroxymonosulfate activation for sulfamethazine degradation by ultrasound irradiation: Performances and mechanisms. Chem. Eng. J. 2018, 335, 145–153. [Google Scholar] [CrossRef]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S.; Li, X.; Wiesner, M.R.; Fang, J. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Jafry, A.T.; Kang, S.B.; Seo, J.Y.; Baek, K.Y.; Kim, E.J.; Pan, J.G.; Choi, J.Y.; Kim, H.J.; Lee, K.H.; et al. Organophosphorus hydrolase-poly-beta-cyclodextrin as a stable self-decontaminating bio-catalytic material for sorption and degradation of organophosphate pesticide. J. Hazard. Mater. 2019, 365, 261–269. [Google Scholar] [CrossRef]
- Chen, C.; Ma, T.; Shang, Y.; Gao, B.; Jin, B.; Dan, H.; Li, Q.; Yue, Q.; Li, Y.; Wang, Y.; et al. In-situ pyrolysis of Enteromorpha as carbocatalyst for catalytic removal of organic contaminants: Considering the intrinsic N/Fe in Enteromorpha and non-radical reaction. Appl. Catal. B-Environ. 2019, 250, 382–395. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Hu, Z.-T.; Sun, Y.-M.; Webster, R.D.; Li, S.-Z.; Lim, T.-T. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl. Catal. B-Environ. 2018, 225, 243–257. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lisak, G.; Webster, R.D.; Liang, Y.-N.; Veksha, A.; Giannis, A.; Moo, J.G.S.; Lim, J.-W.; Lim, T.-T. Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst: Durability of surface active sites. Appl. Catal. B-Environ. 2018, 233, 120–129. [Google Scholar] [CrossRef]
- Huang, Y.M.; Li, G.; Li, M.; Yin, J.; Meng, N.; Zhang, D.; Cao, X.Q.; Zhu, F.P.; Chen, M.; Li, L.; et al. Kelp-derived N-doped biochar activated peroxymonosulfate for ofloxacin degradation. Sci. Total Environ. 2021, 754, 141999. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Zhao, X. Heterogeneous degradation of refractory pollutants by peroxymonosulfate activated by CoOx-doped ordered mesoporous carbon. Chem. Eng. J. 2017, 328, 1112–1121. [Google Scholar] [CrossRef]
- Li, G.; Cao, X.-q.; Meng, N.; Huang, Y.-m.; Wang, X.-d.; Gao, Y.-y.; Li, X.; Yang, T.-s.; Li, B.-l.; Zhang, Y.-z.; et al. Fe3O4 supported on water caltrop-derived biochar toward peroxymonosulfate activation for urea degradation: The key role of sulfate radical. Chem. Eng. J. 2022, 433, 133595. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Zhou, X.; Zhang, C.; Chen, L.; Yan, H.; Qin, L.; Huang, D.; Ye, H.; Chen, W.; et al. Peroxydisulfate activation by sulfur-doped ordered mesoporous carbon: Insight into the intrinsic relationship between defects and 1O2 generation. Water Res. 2022, 221, 118797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Ma, S.; Zhao, P.; Xu, S.; Zhan, S. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: Structure dependence and mechanism. Chem. Eng. J. 2019, 360, 157–170. [Google Scholar] [CrossRef]
- Wang, L.; Lan, X.; Peng, W.; Wang, Z. Uncertainty and misinterpretation over identification, quantification and transformation of reactive species generated in catalytic oxidation processes: A review. J. Hazard. Mater. 2021, 408, 124436. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, G.; Xian, G.; Ren, Z.; Wei, T.; Li, Q.; Zhang, Y.; Zou, Z. Tetracycline degradation by persulfate activated with magnetic γ-Fe2O3/CeO2 catalyst: Performance, activation mechanism and degradation pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation. J. Hazard. Mater. 2020, 397, 122764. [Google Scholar] [CrossRef]
- Xu, X.; Qin, J.; Wei, Y.; Ye, S.; Shen, J.; Yao, Y.; Ding, B.; Shu, Y.; He, G.; Chen, H. Heterogeneous activation of persulfate by NiFe2−xCoxO4-RGO for oxidative degradation of bisphenol A in water. Chem. Eng. J. 2019, 365, 259–269. [Google Scholar] [CrossRef]
- Liu, D.; Li, M.; Li, X.; Ren, F.; Sun, P.; Zhou, L. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation. Chem. Eng. J. 2020, 387, 124008. [Google Scholar] [CrossRef]
- Liu, B.; Song, W.; Zhang, W.; Zhang, X.; Pan, S.; Wu, H.; Sun, Y.; Xu, Y. Fe3O4@CNT as a high-effective and steady chainmail catalyst for tetracycline degradation with peroxydisulfate activation: Performance and mechanism. Sep. Purif. Technol. 2021, 273, 118705. [Google Scholar] [CrossRef]
- Shao, F.; Wang, Y.; Mao, Y.; Shao, T.; Shang, J. Degradation of tetracycline in water by biochar supported nanosized iron activated persulfate. Chemosphere 2020, 261, 127844. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Yuan, X.; Wu, Z.; Jiang, L.; Zhang, J.; Li, Y.; Zeng, G.; Mo, D. Efficient degradation of tetracycline by heterogeneous cobalt oxide/cerium oxide composites mediated with persulfate. Sep. Purif. Technol. 2019, 212, 223–232. [Google Scholar] [CrossRef]
- Ouyang, M.; Li, X.; Xu, Q.; Tao, Z.; Yao, F.; Huang, X.; Wu, Y.; Wang, D.; Yang, Q.; Chen, Z.; et al. Heterogeneous activation of persulfate by Ag doped BiFeO3 composites for tetracycline degradation. J. Colloid Interface Sci. 2020, 566, 33–45. [Google Scholar] [CrossRef]
- Liu, T.; Wu, K.; Wang, M.; Jing, C.; Chen, Y.; Yang, S.; Jin, P. Performance and mechanisms of sulfadiazine removal using persulfate activated by Fe3O4@CuOx hollow spheres. Chemosphere 2021, 262, 127845. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, S.; Su, Y.; Li, T.; Ma, T.; Ren, H. Hierarchically fusiform CuO microstructures decorated with Fe3O4 nanoparticles as novel persulfate activators for 4-aminobenzenesulfonic acid degradation in aqueous solutions. J. Alloys Compd. 2020, 815, 152394. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, D.; Choi, J.; Lee, H.; Seo, J.; Kim, T.; Lee, K.M.; Pham, A.L.; Lee, C. Nickel-Nickel oxide nanocomposite as a magnetically separable persulfate activator for the nonradical oxidation of organic contaminants. J. Hazard. Mater. 2020, 388, 121767. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, J.; Li, C.; Liao, Q.; Xiao, R.; Yang, W. Strong synergistic effect of Co3O4 encapsulated in nitrogen-doped carbon nanotubes on the nonradical-dominated persulfate activation. Carbon 2020, 158, 172–183. [Google Scholar] [CrossRef]
- Wang, J.; Kou, L.; Zhao, L.; Duan, W. One-pot fabrication of sludge-derived magnetic Fe, N-codoped carbon catalysts for peroxymonosulfate-induced elimination of phenolic contaminants. Chemosphere 2020, 248, 126076. [Google Scholar] [CrossRef]
- Miao, W.; Liu, Y.; Wang, D.; Du, N.; Ye, Z.; Hou, Y.; Mao, S.; Ostrikov, K. The role of Fe-Nx single-atom catalytic sites in peroxymonosulfate activation: Formation of surface-activated complex and non-radical pathways. Chem. Eng. J. 2021, 423, 130250. [Google Scholar] [CrossRef]
- Dung, N.T.; Trang, T.T.; Thao, V.D.; Thu, T.V.; Tung, N.Q.; Huy, N.N. Enhanced degradation of organic dyes by peroxymonosulfate with Fe3O4-CoCO3/rGO hybrid activation: A comprehensive study. J. Taiwan Inst. Chem. Eng. 2022, 133, 104279. [Google Scholar] [CrossRef]
- Li, J.; Lin, Q.; Luo, H.; Fu, H.; Wu, L.; Chen, Y.; Ma, Y. The effect of nanoscale zero-valent iron-loaded N-doped biochar on the generation of free radicals and nonradicals by peroxydisulfate activation. J. Water Process Eng. 2022, 47, 102681. [Google Scholar] [CrossRef]
- Gu, M.; Farooq, U.; Lu, S.; Zhang, X.; Qiu, Z.; Sui, Q. Degradation of trichloroethylene in aqueous solution by rGO supported nZVI catalyst under several oxic environments. J. Hazard. Mater. 2018, 349, 35–44. [Google Scholar] [CrossRef]
- Ahmad, M.; Teel, A.L.; Watts, R.J. Mechanism of persulfate activation by phenols. Environ. Sci. Technol. 2013, 47, 5864–5871. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, C.; Zhang, P.; Yue, Q.; Li, Y.; Gao, B.; Xu, X. Removal of sulfamethoxazole from water via activation of persulfate by Fe3C@NCNTs including mechanism of radical and nonradical process. Chem. Eng. J. 2019, 375, 122004. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Qian, Z.; Ouyang, T.; Sun, H.; Qin, J.; Tadé, M.O.; Wang, S. Bread-making synthesis of hierarchically Co@C nanoarchitecture in heteroatom doped porous carbons for oxidative degradation of emerging contaminants. Appl. Catal. B-Environ. 2018, 225, 76–83. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Duan, X.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S. N-Doped Graphene from Metal–Organic Frameworks for Catalytic Oxidation of p-Hydroxylbenzoic Acid: N-Functionality and Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 2693–2701. [Google Scholar] [CrossRef]
- Wang, J.; Duan, X.; Gao, J.; Shen, Y.; Feng, X.; Yu, Z.; Tan, X.; Liu, S.; Wang, S. Roles of structure defect, oxygen groups and heteroatom doping on carbon in nonradical oxidation of water contaminants. Water Res. 2020, 185, 116244. [Google Scholar] [CrossRef]
- Luo, R.; Li, M.; Wang, C.; Zhang, M.; Khan, M.A.N.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res. 2019, 148, 416–424. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B-Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Yun, E.T.; Yoo, H.Y.; Bae, H.; Kim, H.I.; Lee, J. Exploring the Role of Persulfate in the Activation Process: Radical Precursor Versus Electron Acceptor. Environ. Sci. Technol. 2017, 51, 10090–10099. [Google Scholar] [CrossRef] [PubMed]
- Idrees, A.; Shan, A.; Ali, M.; Abbas, Z.; Shahzad, T.; Hussain, S.; Mahmood, F.; Farooq, U.; Danish, M.; Lyu, S. Highly efficient degradation of trichloroethylene in groundwater based on persulfate activation by polyvinylpyrrolidone functionalized Fe/Cu bimetallic nanoparticles. J. Environ. Chem. Eng. 2021, 9, 105341. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Huang, W.; Xue, C.; Zhu, Y.; Wang, Q.; Liu, D. Innovatively employing magnetic CuO nanosheet to activate peroxymonosulfate for the treatment of high-salinity organic wastewater. J. Environ. Sci. 2020, 88, 46–58. [Google Scholar] [CrossRef]
- Soubh, A.M.; Baghdadi, M.; Abdoli, M.A.; Aminzadeh, B. Zero-valent iron nanofibers (ZVINFs) immobilized on the surface of reduced ultra-large graphene oxide (rULGO) as a persulfate activator for treatment of landfill leachate. J. Environ. Chem. Eng. 2018, 6, 6568–6579. [Google Scholar] [CrossRef]
- Karimipourfard, D.; Eslamloueyan, R.; Mehranbod, N. Heterogeneous degradation of stabilized landfill leachate using persulfate activation by CuFe2O4 nanocatalyst: An experimental investigation. J. Environ. Chem. Eng. 2020, 8, 103426. [Google Scholar] [CrossRef]
- Guo, R.; Meng, Q.; Zhang, H.; Zhang, X.; Li, B.; Cheng, Q.; Cheng, X. Construction of Fe2O3/Co3O4/exfoliated graphite composite and its high efficient treatment of landfill leachate by activation of potassium persulfate. Chem. Eng. J. 2019, 355, 952–962. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, X.; Wang, D.; Wang, H.; Wu, Z.; Jiang, L.; Mo, D.; Yang, G.; Guan, R.; Zeng, G. Recyclable zero-valent iron activating peroxymonosulfate synchronously combined with thermal treatment enhances sludge dewaterability by altering physicochemical and biological properties. Bioresour. Technol. 2018, 262, 294–301. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Tian, J.; Zhang, H.; Li, F.; Pei, J. Enhanced dewaterability of waste activated sludge by UV assisted ZVI-PDS oxidation. J. Environ. Sci. 2022, 113, 152–164. [Google Scholar] [CrossRef]
- Liu, C. Enhancement of dewaterability and heavy metals solubilization of waste activated sludge conditioned by natural vanadium-titanium magnetite-activated peroxymonosulfate oxidation with rice husk. Chem. Eng. J. 2019, 359, 217–224. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Zhang, T.; Nie, Y.; Shi, W.; Ai, S. Iron nanoparticles supported on N-doped carbon foam with honeycomb microstructure: An efficient potassium peroxymonosulfate activator for the degradation of fluoranthene in water and soil. Chemosphere 2022, 286, 131603. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, S.; Liang, W.; Li, C.; Nie, Y.; Dong, J.; Shi, W.; Ai, S. A low-cost and eco-friendly powder catalyst: Iron and copper nanoparticles supported on biochar/geopolymer for activating potassium peroxymonosulfate to degrade naphthalene in water and soil. Chemosphere 2022, 303, 135185. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, J.; Liu, X.; Lin, C.; Sun, K.; Zhang, H.; Li, X.; Fan, G. Activation of peroxydisulfate by nanoscale zero-valent iron for sulfamethoxazole removal in agricultural soil: Effect, mechanism and ecotoxicity. Chemosphere 2019, 223, 196–203. [Google Scholar] [CrossRef]
- Bandala, E.R.; Liu, A.; Wijesiri, B.; Zeidman, A.B.; Goonetilleke, A. Emerging materials and technologies for landfill leachate treatment: A critical review. Environ. Pollut. 2021, 291, 118133. [Google Scholar] [CrossRef]
- Viegas, C.; Nobre, C.; Mota, A.; Vilarinho, C.; Gouveia, L.; Gonçalves, M. A circular approach for landfill leachate treatment: Chemical precipitation with biomass ash followed by bioremediation through microalgae. J. Environ. Chem. Eng. 2021, 9, 105187. [Google Scholar] [CrossRef]
- Karimipourfard, D.; Eslamloueyan, R.; Mehranbod, N. Novel heterogeneous degradation of mature landfill leachate using persulfate and magnetic CuFe2O4/RGO nanocatalyst. Process Saf. Environ. 2019, 131, 212–222. [Google Scholar] [CrossRef]
- Maqbool, T.; Cho, J.; Hur, J. Improved dewaterability of anaerobically digested sludge and compositional changes in extracellular polymeric substances by indigenous persulfate activation. Sci. Total Environ. 2019, 674, 96–104. [Google Scholar] [CrossRef]
- Wang, H.F.; Ma, Y.J.; Wang, H.J.; Hu, H.; Yang, H.Y.; Zeng, R.J. Applying rheological analysis to better understand the mechanism of acid conditioning on activated sludge dewatering. Water Res. 2017, 122, 398–406. [Google Scholar] [CrossRef]
- Wang, D.; Liu, X.; Zeng, G.; Zhao, J.; Liu, Y.; Wang, Q.; Chen, F.; Li, X.; Yang, Q. Understanding the impact of cationic polyacrylamide on anaerobic digestion of waste activated sludge. Water Res. 2018, 130, 281–290. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Wang, D.; Yang, G.; Pan, L.; Wang, Q.; Ni, B.J.; Li, H.; Yuan, X.; Jiang, L.; et al. Fe(II) catalyzing sodium percarbonate facilitates the dewaterability of waste activated sludge: Performance, mechanism, and implication. Water Res. 2020, 174, 115626. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zeng, L.; Huang, J.; Mo, Z.; Guan, Z.; Sun, S.; Liang, J.; Huang, S. Enhanced sludge dewaterability by a novel MnFe2O4-Biochar activated peroxymonosulfate process combined with Tannic acid. Chem. Eng. J. 2022, 429, 132280. [Google Scholar] [CrossRef]
- Gou, Y.; Zhao, Q.; Yang, S.; Qiao, P.; Cheng, Y.; Song, Y.; Sun, Z.; Zhang, T.; Wang, L.; Liu, Z. Enhanced degradation of polycyclic aromatic hydrocarbons in aged subsurface soil using integrated persulfate oxidation and anoxic biodegradation. Chem. Eng. J. 2020, 394, 125040. [Google Scholar] [CrossRef]





| Quench Agents | Targeted ROS | Reaction Rate Constants (M−1·s−1) | Identification of ROS | Location of ROS | Ref. |
|---|---|---|---|---|---|
| MeOH | SO4·− | 3.2 × 106 | SO4·−, ·OH | In the solution | [60,61,62] |
| ·OH | 9.7 × 108 | ||||
| TBA | SO4·− | 4–9.1 × 105 | ·OH | In the solution | |
| ·OH | 3.8–7.6 × 108 | ||||
| EtOH | SO4·− | 1.6–7.7 × 107 | SO4·−, ·OH | In the solution | [63,64] |
| ·OH | 1.2–2.8 × 108 | ||||
| Phenol | SO4·− | 8.8 × 109 | SO4·−, ·OH | Catalyst surface | [65,66] |
| ·OH | 6.6 × 109 | ||||
| KI | SO4·− | - | SO4·−, ·OH | Catalyst surface | [61,67] |
| ·OH | - | ||||
| NB | SO4·− | <106 | ·OH | Catalyst surface | [59,68,69] |
| ·OH | 3.9 × 109 | ||||
| p-BQ | O2·− | 1.0 × 109 | O2·− | Catalyst surface | [66,70] |
| L-listidine | 1O2 | 3.2 × 107 | 1O2 | - | [71,72] |
| FFA | 1.2 × 108 | [73,74] | |||
| NaN3 | 1.0 × 109 | [66,75] | |||
| NaClO4 | e− | - | e− | - | [59,68,76] |
| Reaction Pathway | System | Targeted Pollutant | pH | Quenching Agent (Degradation Rate after Inhibition, Control Degradation Rate) | EPR Signal | Activation Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Radical pathway | Fe3O4-BC/PMS | BPA | 3.0 | TBA (84.5%, 100%) EtOH (47.6%, 100%) | DMPO-X | SO4·− (major), ·OH | [35] |
| nZVI-Omt/PDS | SMZ | 6.8 | TBA (73%, 97%) MeOH (40%, 97%) | DMPO-·OH DMPO-SO4·− | SO4·−, ·OH | [37] | |
| nZVI-BC/PDS | TC | 5.0 | TBA (75.95%, 87.58%) EtOH (32.65%, 87.58%) | DMPO-·OH DMPO-SO4·− | SO4·− (major), ·OH | [83] | |
| Co3O4-CeO2/PDS | TC | 7 | TBA (52%, 79%) MeOH (41%, 79%) | DMPO-·OH DMPO-SO4·− | SO4·−, ·OH(major) | [84] | |
| Co3O4-Bi2O3@NF/PMS | BPA | 3.4 | TBA (~95%, 95.6%) MeOH (~95%, 95.6%) KI (~8%, 95.6%) | DMPO-·OH DMPO-SO4·− | SO4·−surface, ·OHsurface | [53] | |
| Ag0.4-BiFeO3/PDS | TC | 4.5 | TBA (~48%, 91%) MeOH (~52%, 91%) | DMPO-·OH DMPO-SO4·− | SO4·−, ·OH(major) | [85] | |
| Fe3O4@CuOx/PDS | sulfadiazine (SDZ) | 7 | TBA (58.6%, 95%) MeOH (18.2%, 95%) | DMPO-·OH DMPO-SO4·− | SO4·− (major), ·OH | [86] | |
| Fe3O4/hf-CuO/PDS | 4-aminobenzenesulfonic acid (4-ABS) | 7 | TBA (45.7%, 90%) MeOH (4.3%, 90%) | DMPO-·OH DMPO-SO4·− | SO4·−, ·OH | [87] | |
| Fe3O4/MW/PDS | PNP | 3.4 | TBA (76.2%, 98.2%) MeOH (29.3%, 98.2%) | DMPO-·OH DMPO-SO4·− | SO4·− (major), ·OH | [34] | |
| Non-radical pathway | RC/CNTs/Fe3O4 NPs/PDS | BPA | 6.07 | TBA (~100%, 100%) EtOH (~100%, 100%) FFA (~60%, 100%) | TEMP-1O2 | 1O2, electron transfer, catalyst-PDS * | [55] |
| rGO-Fe3O4/PDS | NOR | 6.47 | TBA (~82%, 89.6%) EtOH (~73%, 89.6%) FFA (~50%, 89.6%) | - | 1O2, electron transfer | [33] | |
| Ni-NiO/PDS | 4-CP | 7.0 | MeOH (~80%, 100%) (The removal of FFA is lower than 10%) | - | Electron transfer | [88] | |
| Co3O4@NCNTs/PDS | Orange G (OG) | 7.0 | TBA (90.1%, 100%) EtOH (~90%, 100%) FFA (17.3%, 100%) p-BQ (~70%, 100%) | DMPO-·OH DMPO-SO4·− DMPO-O2·− TEMP-1O2(major) | 1O2(major), electron transfer, catalyst-PDS * | [89] | |
| UBC-x/PMS | BPA | 6.84 | TBA (~100%, 100%) EtOH (~100%, 100%) FFA (75%, 100%) | DMPO-·OH DMPO-SO4·− DMPO-O2·− TEMP-1O2 | 1O2(major) | [90] | |
| Radical pathway and non-radical pathway | Fe3O4/MC/PMS | HBA | - | Phenol (10%, 100%) NB (88%, 100%) p-BQ (67%, 100%) NaClO4 (70%, 100%) | DMPO-X | SO4·−-surface, ·OH, O2·−, electron transfer | [59] |
| Fe-N-BC/PDS | acid orange (AO7) | 7.0 | TBA (56.9%, 98.2%) MeOH (44.6%, 98.2%) KI (~30%, 98.2%) FFA (~68%, 98.2%) BQ (~35%, 98.2%) | DMPO-·OH DMPO-SO4·− DMPO-O2·− TEMP-1O2 | SO4·−, ·OH, O2·−, 1O2, electron transfer, catalyst-PDS * | [79] | |
| FeCNx/PMS | BPA | 6.5 | MeOH (~90%, 94%) KI (~42%, 94%) NaN3 (~16%, 94%) | DMPO-·OH DMPO-SO4·− TEMP-1O2 | SO4·−-surface, ·OH-surface, 1O2, electron transfer, catalyst-PMS * | [91] | |
| Fe3O4/CoCO3/rGO/PMS | Rhodamine B (RhB) | 7.0 | TBA (72.5%, 98.69%) EtOH (10.29%, 98.69%) FFA (complete inhibition) | - | SO4·− (major), 1O2(major), ·OH | [92] | |
| nZVI@NBC/PDS | BPA | 7.0 | TBA (77.3%, 100%) EtOH (74.1%, 100%) FFA (12%, 100%) | DMPO-·OH DMPO-SO4·− TEMP-1O2 | SO4·−, ·OH, 1O2 | [93] |
| Practical Field | Reaction System | Reaction Conditions | Results | Refs. |
|---|---|---|---|---|
| Actual wastewater | PVP-NZVI-Cu/PDS | [PDS] = 18 mmol/L; [catalyst] = 1.2 g/L; [TCE] = 0.15 mmol/L; pH = 3.2; t = 1 h | 99.5% of TCE removal | [103] |
| Mag-CuO/PMS | [Na2SO4] = 0.2 mol/L; [PMS] = 2 mmol/L; [catalyst] = 0.1 g/L; pH = 7; t = 30 min; [AO7] = 0.2 mmol/L, [MB] = [RhB] = [ATZ] = 0.1 mmol/L | The removal efficiencies of AO7, MB, RhB, and ATZ were 95.81%, 74.57%, 100%, and 100%, respectively. | [104] | |
| Landfill leachate | ZVINFS/rULGO/PDS | [PDS/COD] = 3; [catalyst] = 1.6 g/L; pH = 3; t = 45 min | 80.87% removal of COD and 72.38% of NH3 removal | [105] |
| CuFe2O4/PDS | [PDS] = 5 g/L; [catalyst] = 1.5 g/L; pH = 2; t = 60 min | 57% removal of COD, 71% removal of NH3-N and 63% of color, respectively. | [106] | |
| Fe2O3/Co3O4/EG/PDS | [PDS] = 0.05 mol/L; [catalyst] = 0.1 g; pH = 5; t = 60 min | 90.6% removal of COD and 67.1% of NH4+-N removal | [107] | |
| Biological waste sludge | ZVI/PMS | [PMS] = 24.5 mg/g TSS; [catalyst] = 260.7 mg/g TSS | SRF decreased by 83.6% | [108] |
| UV/ZVI/PDS | [nZVI/PDS] = 0.6; [PDS] = 200 mg/gTSS; UV = 254 nm; pH = 6.54; t = 20 min | 64.0% decreased of CST and 78.2% decreased of SRF | [109] | |
| VTM/RH/PMS | [PMS] = 200 mg/g TSS; [VTM] = 1 g/g TSS; [RH] = 200 mg/g TSS | 94.8% reduction of CST and 63.4% of Wc | [110] | |
| Soil | Fe@CF-N/PMS | [PMS] = 0.2 mmol/L; [catalyst] = 25 mg; pH = 5; t = 180 min; [FLT] = 10 mg/L | 78.12% removal of FLT | [111] |
| Fe-Cu@BC-GM/PMS | [PMS] = 100 mg/L; [catalyst] = 100 mg/L; pH = 3; t = 120 min; [NAP] = 10 mg/L | 67.98% removal of NAP | [112] | |
| nZVI/PDS | [nSMX/PDS] = 1/75; [catalyst] = 0.03 g/g soil; [soil/water] = 1/1 | Removal efficiencies of SMX were 87.6% (cinnamon soil), 90.6% (yellow brown earths), 80.8% (brown earths), 86.5% (black soils), and 96.1% (red earths), respectively. | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, K.; Shi, F.; Cao, M.; Zheng, Q.; Zhang, G. A Review of Persulfate Activation by Magnetic Catalysts to Degrade Organic Contaminants: Mechanisms and Applications. Catalysts 2022, 12, 1058. https://doi.org/10.3390/catal12091058
Tian K, Shi F, Cao M, Zheng Q, Zhang G. A Review of Persulfate Activation by Magnetic Catalysts to Degrade Organic Contaminants: Mechanisms and Applications. Catalysts. 2022; 12(9):1058. https://doi.org/10.3390/catal12091058
Chicago/Turabian StyleTian, Ke, Fengyin Shi, Menghan Cao, Qingzhu Zheng, and Guangshan Zhang. 2022. "A Review of Persulfate Activation by Magnetic Catalysts to Degrade Organic Contaminants: Mechanisms and Applications" Catalysts 12, no. 9: 1058. https://doi.org/10.3390/catal12091058
APA StyleTian, K., Shi, F., Cao, M., Zheng, Q., & Zhang, G. (2022). A Review of Persulfate Activation by Magnetic Catalysts to Degrade Organic Contaminants: Mechanisms and Applications. Catalysts, 12(9), 1058. https://doi.org/10.3390/catal12091058