Tailorable Formation of Hierarchical Structure Silica (HMS) and Its Application in Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussions
2.1. Formation Mechanism of HMS Materials
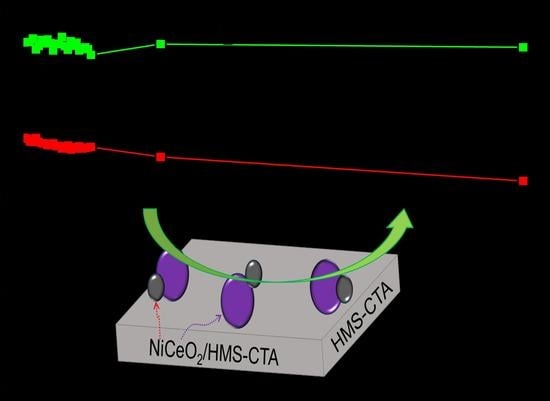
2.2. Catalytic Activity of Ni Based Catalysts in DRM Reaction
3. Materials and Methods
3.1. Materials
3.2. Materials Preparation
3.3. Catalytic Activity Testing
3.4. Materials Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, Y.F.; Li, Y.Z.; Zhang, C.; Zhao, T.Y.; Tuo, X.C.; Guo, J.; Gong, Y.M. Formaldehyde-controlled synthesis of multishelled hollow mesoporous SiO2 microspheres. Langmuir 2019, 35, 14517–14521. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Fu, Z.Y.; Su, B.L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.D.; Deng, T.; Zhao, D.Y.; Feng, P.Y.; Pine, D.; Chmelka, B.F.; Whitesides, G.M.; Stucky, G.D. Hierarchically ordered oxides. Science 1998, 282, 2244–2246. [Google Scholar] [CrossRef] [PubMed]
- Todorova, S.; Blin, J.L.; Naydenov, A.; Lebeau, B.; Karashanova, D.; Kolev, H.; Gaudin, P.; Velinova, R.; Vidal, L.; Michelin, L.; et al. Co-Mn oxides supported on hierarchical macro-mesoporous silica for CO and VOCs oxidation. Catal. Today 2021, 361, 94–101. [Google Scholar] [CrossRef]
- Qin, L.M.; Niu, D.C.; Li, N.; Luo, X.F.; Qin, X.; Chen, J.Z.; Li, Y.S.; Shi, J.L. Hydrophobicity-induced electrostatic interfacial self-assembly for porous silica nanospheres with tunable pore sizes and pore hierarchies. Chem. Eng. J. 2021, 405, 126936. [Google Scholar] [CrossRef]
- Kerkhofs, S.; Willhammar, T.; Noortgate, H.V.D.; Kirschhock, C.E.A.; Breynaert, E.; Tendeloo, G.V.; Bals, S.; Martens, J.A. Self-Assembly of pluronic F127—silica spherical core–shell nanoparticles in cubic close-packed structures. Chem. Mater. 2015, 27, 5161–5169. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.W.; Chang, F.C. Self-assembly of an A–B diblock copolymer blended with a C homopolymer and a C–D diblock copolymer through hydrogen bonding interaction. Polymer 2010, 51, 4176–4184. [Google Scholar] [CrossRef]
- Shi, C.X.; Du, G.; Wang, J.G.; Sun, P.C.; Chen, T.H. Polyelectrolyte–surfactant mesomorphous complex templating: A versatile approach for hierarchically porous materials. Langmuir 2020, 36, 1851–1863. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, R.B.; Kuo, S.W. Phase behavior of hierarchical mesoporous silicas prepared using ABC triblock copolymers as single templates. RSC Adv. 2013, 3, 17411–17423. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Atayde, E.C., Jr.; Matsagar, B.M.; Na, J.; Yamauchi, Y.; Wu, K.C.W.; Kuo, S.W. Construction hierarchically mesoporous/microporous materials based on block copolymer and covalent organic framework. J. Taiwan Inst. Chem. Eng. 2020, 112, 180–192. [Google Scholar] [CrossRef]
- Esquena, J.; Nestor, J.; Vílchez, A.; Aramaki, K.; Solans, C. Preparation of mesoporous/macroporous materials in highly concentrated emulsions based on cubic phases by a single-step method. Langmuir 2012, 28, 12334–12340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; He, M.; Zhou, Y.M.; Sheng, X.L.; Fu, X.Q.; Zhang, Y.W. Synthesis of micro/mesoporous silica material by dual-template method as a heterogeneous catalyst support for alkylation. RSC Adv. 2015, 5, 28124–28132. [Google Scholar] [CrossRef]
- Liu, C.C.; Li, J.G.; Kuo, S.W. Co-template method provides hierarchical mesoporous silicas with exceptionally ultra-low refractive indices. RSC Adv. 2014, 4, 20262–20272. [Google Scholar] [CrossRef]
- Stein, A.; Rudisill, S.G.; Petkovich, N.D. Perspective on the influence of interactions between hard and soft templates and precursors on morphology of hierarchically structured porous materials. Chem. Mater. 2013, 26, 259–276. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Deng, Y.H.; Wei, J.; Sun, Z.K.; Gu, D.; Bongard, H.; Liu, C.; Wu, H.H.; Tu, B.; Schüth, F.; et al. Design of amphiphilic ABC triblock copolymer for templating synthesis of large-pore ordered mesoporous carbons with tunable pore wall thickness. Chem. Mater. 2009, 21, 3996–4005. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, R.B.; Kuo, S.W. Hierarchical mesoporous silica fabricated from an ABC triblock terpolymer as a single template. Macromol. Rapid Comm. 2012, 33, 678–682. [Google Scholar] [CrossRef]
- Li, L.M.; Liu, D.P.; Guo, Z.L.; Liu, Y.; Chu, W. Improved facile synthesis of mesoporous SBA-15-CTA using citric acid under mild conditions. J. Solid State Chem. 2020, 282, 121079. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Mathe, V.L. Photocatalytic hydrogen production using TiO2 nanogranules prepared by hydrothermal route. Chem. Phys. Lett. 2019, 731, 136582. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W. Porous nanoplate-like tungsten trioxide/reduced graphene oxide catalyst for sonocatalytic degradation and photocatalytic hydrogen production. Surf. Interfaces 2021, 24, 101075. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W. Spongy ball-like copper oxide nanostructure modified by reduced graphene oxide for enhanced photocatalytic hydrogen production. Mater. Res. Bull. 2021, 133, 111026. [Google Scholar] [CrossRef]
- Hinsch, J.J.; Liu, J.; White, J.J.; Wang, Y. The Role of steps on silver nanoparticles in electrocatalytic oxygen reduction. Catalysts 2022, 12, 576. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Pimerzin, A.; Nedolivko, V.; Zasypalov, G.; Stytsenko, V.; Karakhanov, E.; Vinokurov, V. Ruthenium catalysts templated on mesoporous MCM-41 type silica and natural clay nanotubes for hydrogenation of benzene to cyclohexane. Catalysts 2020, 10, 537. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrog. Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Tang, S.; Xu, Y.S.; Zhang, W.D. Embedding thiophene-amide into g-C3N4 skeleton with induction and delocalization effects for high photocatalytic H2 evolution. Catalysts 2022, 12, 1043. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sust. Energ. Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Ekeoma, B.C.; Yusuf, M.; Johari, K.; Abdullah, B. Mesoporous silica supported Ni-based catalysts for methane dry reforming: A review of recent studies. Int. J. Hydrog. Energy 2022. [Google Scholar] [CrossRef]
- La Parola, V.; Liotta, L.F.; Pantaleo, G.; Testa, M.L.; Venezia, A.M. CO2 reforming of CH4 over Ni supported on SiO2 modified by TiO2 and ZrO2: Effect of the support synthesis procedure. Appl. Catal. A-Gen. 2022, 642, 118704. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, B.; Kattel, S.; Lee, J.H.; Yao, S.; Wu, Q.; Rui, N.; Gomez, E.; Liu, Z.; Xu, W.; et al. Dry reforming of methane over CeO2-supported Pt-Co catalysts with enhanced activity. Appl. Catal. B-Environ. 2018, 236, 280–293. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Jiang, F.; Xu, Y.; Liu, B.; Liu, X.H. Insight into the anti-coking ability of NiM/SiO2 (M=ZrO2, Ru) catalyst for dry reforming of CH4 to syngas. Int. J. Hydrog. Energy 2022, 47, 2268–2278. [Google Scholar] [CrossRef]
- Li, L.M.; Liu, D.P.; Guo, Z.L.; Xi, S.B.; Chu, W.; Liu, Y. Insights into Ni and (Ce)SBA-15-CTA interaction and syngas formation rate. Mol. Catal. 2021, 514, 111850. [Google Scholar] [CrossRef]
- Qiu, C.T.; Wang, S.; Zuo, J.D.; Zhang, B. Photocatalytic CO2 reduction coupled with alcohol oxidation over porous carbon nitride. Catalysts 2022, 12, 672. [Google Scholar] [CrossRef]
- Wang, W.; Wu, C.; Sun, R.; Li, D.; Ru, H.Q. Simple and controllable preparation of SBA-15 microspheres by poly(vinyl alcohol)-assisted P123 templating system. Micropor. Mesopor. Mater. 2020, 302, 110211. [Google Scholar] [CrossRef]
- Guo, W.P.; Su, F.; Zhao, X.S. Ordered mesostructured carbon templated by SBA-16 silica. Carbon 2005, 43, 2423–2426. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.B.; Zou, R.Q.; Zhao, Y.L. Heterogeneous catalysis in zeolites, mesoporous silica, and metal-organic frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Du, G.; Song, Y.; Li, N.; Xu, L.J.; Tong, C.; Feng, Y.H.; Chen, T.H.; Xu, J.H. Cage-like hierarchically mesoporous hollow silica microspheres templated by mesomorphous polyelectrolyte-surfactant complexes for noble metal nanoparticles immobilization. Colloids Surf. A 2019, 575, 129–139. [Google Scholar] [CrossRef]
- Wang, F.G.; Han, K.H.; Yu, W.S.; Zhao, L.; Wang, Y.; Wang, X.J.; Yu, H.; Shi, W.D. Low temperature CO2 reforming with methane reaction over CeO2-modified Ni@SiO2 catalysts. ACS App. Mater. Interfaces 2020, 31, 35022–35034. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.Y.; Wang, X.; Ma, H.Y.; Assabumrungrat, S.; Gong, J.L. Ceria-promoted Ni-SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B-Environ. 2015, 176, 532–541. [Google Scholar] [CrossRef]
- Liang, T.Y.; Low, P.Y.; Lin, Y.S.; Tsai, D.H. Spherical porous nanoclusters of NiO and CeO2 nanoparticles as catalysts for syngas production. ACS Appl. Nano Mater. 2020, 3, 9035–9045. [Google Scholar] [CrossRef]
- Guo, Y.; Zou, J.M.; Shi, X.; Rukundo, P.; Wang, Z.J. A Ni-CeO2−CDC-SiC catalyst with improved coke resistance in CO2 reforming of methane. ACS Sustain. Chem. Eng. 2017, 5, 2330–2338. [Google Scholar] [CrossRef]
- Lanre, M.S.; Abasaeed, A.E.; Fakeeha, A.H.; Ibrahim, A.A.; Al-Awadi, A.S.; Jumah, A.B.; Al-Mubaddel, F.S.; Al-Fatesh, A.S. Lanthanum–cerium-modified nickel catalysts for dry reforming of methane. Catalysts 2022, 12, 715. [Google Scholar] [CrossRef]
- Yan, X.L.; Hu, T.; Liu, P.; Li, S.; Zhao, B.R.; Zhang, Q.; Jiao, W.Y.; Chen, S.; Wang, P.F.; Lu, J.J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane; effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Cataly. B-Environ. 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Zhang, S.H.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane. ACS Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]








| Entry No. | F127 Mass (g) | Citric Concentration (mol/L) | Hydrothermal Temp./Time (°C, h) | pH Value | SBET (m2/g) | Micropore Area (m2/g) | Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 0 | 100, 24 | 6.80 | 332.9 | 29.3 | 0.78 | 10.84 |
| 2 | 2.5 | 0.018 | 100, 24 | 2.57 | 651.1 | 88.8 | 0.95 | 6.80 |
| 3 | 2.5 | 0.055 | 100, 24 | 2.20 | 690.4 | 86.9 | 0.99 | 6.87 |
| 4 | 2.5 | 0.28 | 100, 24 | 1.82 | 739.9 | 86.4 | 0.75 | 4.78 |
| 5 | 2.5 | 0.55 | 100, 24 | 1.63 | 725.8 | 69.6 | 0.78 | 5.21 |
| 6 | 2.5 | 1.03 | 100, 24 | 1.40 | 703.2 | 74.2 | 0.88 | 5.76 |
| 7 | 2.5 | 1.82 | 100, 24 | 1.09 | 782.4 | 142.7 | 0.61 | 3.45 |
| 8 | 2.5 | 0.28 | 60, 24 | 1.82 | 443.2 | 181.6 | 0.29 | 3.18 |
| 9 | 2.5 | 0.28 | 140, 24 | 1.82 | 565.3 | 26.0 | 1.38 | 9.37 |
| 10 | 2.5 | 0.28 | 100, 4 | 1.82 | 502.3 | 167.1 | 0.36 | 3.44 |
| 11 | 2.5 | 0.28 | 100, 8 | 1.82 | 546.2 | 160.2 | 0.46 | 4.12 |
| 12 | 2.5 | 0.28 | 100, 16 | 1.82 | 702.6 | 104.2 | 0.69 | 4.67 |
| 13 | 2.5 | 0.28 | 100, 48 | 1.82 | 792.3 | 30.3 | 0.95 | 5.13 |
| 14 | 2.5 | 0.28 | 100, 72 | 1.82 | 729.7 | 18.2 | 0.96 | 5.39 |
| 15 | 2.5 | 0.28 | 100, 140 | 1.82 | 745.2 | 34.5 | 1.17 | 6.09 |
| 16 | 2.5 | 0.28 | 100, 480 | 1.82 | 629.7 | 55.7 | 1.16 | 8.28 |
| 17 | 0 | 1.03 | 100, 24 | 1.40 | 586.3 | NA | 1.23 | 7.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Deng, J.; Guo, Z.; Chu, W.; Liu, Y. Tailorable Formation of Hierarchical Structure Silica (HMS) and Its Application in Hydrogen Production. Catalysts 2022, 12, 1061. https://doi.org/10.3390/catal12091061
Li L, Deng J, Guo Z, Chu W, Liu Y. Tailorable Formation of Hierarchical Structure Silica (HMS) and Its Application in Hydrogen Production. Catalysts. 2022; 12(9):1061. https://doi.org/10.3390/catal12091061
Chicago/Turabian StyleLi, Luming, Jie Deng, Zhanglong Guo, Wei Chu, and Yan Liu. 2022. "Tailorable Formation of Hierarchical Structure Silica (HMS) and Its Application in Hydrogen Production" Catalysts 12, no. 9: 1061. https://doi.org/10.3390/catal12091061
APA StyleLi, L., Deng, J., Guo, Z., Chu, W., & Liu, Y. (2022). Tailorable Formation of Hierarchical Structure Silica (HMS) and Its Application in Hydrogen Production. Catalysts, 12(9), 1061. https://doi.org/10.3390/catal12091061