The Emergence of the Ubiquity of Cerium in Heterogeneous Oxidation Catalysis Science and Technology
Abstract
:1. Introduction
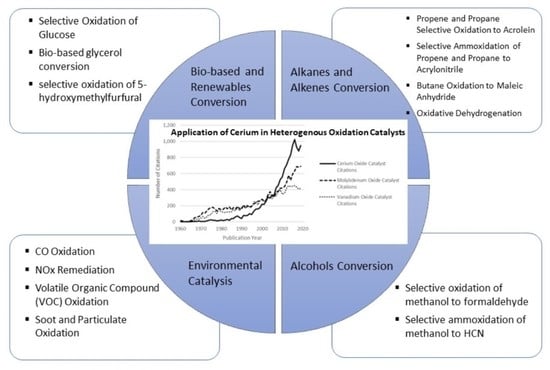
2. Application of Cerium in Oxidation Catalysts
2.1. General
2.2. Selective Oxidation Applications






2.2.1. Propene and Propane Selective Oxidation to Acrolein
- Divalent M2+MoO4 molybdates of the α and/or β structure type in which M2+ is one or more of Ni2+, Co2+, Mg2+, Fe2+ typically as a single solid solution.
- Trivalent Fe2Mo3O12.
2.2.2. Selective Ammoxidation of Propene and Propane to Acrylonitrile
- 1.
- Solid solution/single phase formation within the catalytically active phase.
- 2.
- Synergistic phase interaction (most effectively via structural epitaxy).
2.2.3. Butane Oxidation to Maleic Anhydride
2.2.4. Oxidative Dehydrogenation

- Cerium is directly incorporated into the active phase as evidenced by the X-ray diffraction analysis showing a monotonic increase in unit cell volume of the active M1 crystal phase of MoVNbTeOx (PDF 00-058-0789) with increasing cerium content.
- The redox activity of cerium promotes the stabilization of the high oxidation state of the active catalyst moiety—in this case, the V5+—by promoting the replacement of lattice oxygen in accordance with the operative Mars–van Krevelen mechanism for the reaction.
- Cerium increases the amount of lattice oxygen available, and thus the reducibility of the catalyst, for the reaction, which is manifested in the enhanced activity of the catalyst.
2.2.5. Other Selective Oxidation Reactions
3. Environmental Catalyst Applications
3.1. Combustion/Total Oxidation
3.1.1. CO Oxidation
3.1.2. Volatile Organic Compound (VOC) Oxidation
3.1.3. Soot and Particulate Oxidation—Diesel Engine Exhaust Emission Control
3.2. NOx Remediation
4. Bio-Based and Renewable Chemical Applications
4.1. General
4.2. Select Bio-Based Catalytic Processes
5. Underpinnings for Cerium Oxide Catalysts
5.1. Chemical and Physical Properties of Cerium Oxides Relevant to Catalysis
5.2. Cerium Availability for Catalytic Applications
6. Conclusions and Future Prospects for Cerium Oxide Catalysts
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grasselli, R.K.; Callahan, J.L. Structure-catalytic efficiency relations in uranium-antimony oxide acrylonitrile synthesis catalysts. J. Catal. 1969, 14, 93–103. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Suresh, D.D. Aspects of structure and activity in uranium-antimony oxide acrylonitrile catalysts. J. Catal. 1972, 25, 273. [Google Scholar] [CrossRef]
- Suresh, D.D.; Grasselli, R.K. Uranium Antimonate Catalysts. U.S. Patent 4317747, 2 March 1982. [Google Scholar]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- He, J.-J.; Wang, C.-X.; Zheng, T.-T.; Zhao, Y.-K. Thermally Induced Deactivation and the Corresponding Strategies for Improving Durability in Automotive Three-Way Catalysts. Johns. Matthey Technol. Rev. 2016, 60, 196–203. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Teller, R.G.; Grasselli, R.K.; Kostiner, E. Structural and thermodynamic basis for catalytic behavior of bismuth-cerium molybdate selective oxidation catalysts. ACS Symp. Ser. 1985, 279, 57–74. [Google Scholar] [CrossRef]
- Valange, S.; Védrine, J.C. General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis. Catalysts 2018, 8, 483. [Google Scholar] [CrossRef]
- Eley, D.D.; Rideal, E.K. Parahydrogen conversion on tungsten. Nature 1940, 146, 401. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Kawano, T.; Takahashi, Y.; Fukumoto, N. Catalyst for Producing Unsaturated Aldehyde and/or Unsaturated Carboxylic Acid and Method for Producing Unsaturated Aldehyde and/or Unsaturated Carboxylic Acid Using Catalyst. WO2012036038, 22 March 2012. [Google Scholar]
- Dolhyj, S.R.; Milberger, E.C. Acrylic Acid or Methacrylic Acid. DE 2,448,804, 30 April 1975. [Google Scholar]
- Yunoki, H.; Tanimoto, M.; Nakamura, D. Manufacture of Acrylic Acid by Vapor-Phase Catalytic Oxidation of Acrolein. EP 1,055,662, 29 November 2000. [Google Scholar]
- Aykan, K. Reduction of Bi2O3·MoO3 catalyst during the ammoxidation of propylene in the absence of gaseous oxygen. J. Catal. 1968, 12, 281–290. [Google Scholar] [CrossRef]
- Van den Elzen, A.F.; Rieck, G.D. An outline of the crystal-structure of Bi2Mo2O9. Mater. Res. Bull. 1975, 10, 1163–1168. [Google Scholar] [CrossRef]
- Teller, R.G.; Brazdil, J.F.; Grasselli, R.K. The structure of γ-bismuth molybdate, Bi2MoO6, by powder neutron diffraction. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1984, C40, 2001–2005. [Google Scholar] [CrossRef]
- Sennewald, K.; Vogt, W.; Kandler, J.; Sommerfeld, R.; Sorbe, G. Process for Preparing Unsaturated Nitriles. U.S. Patent 3226422, 28 December 1965. [Google Scholar]
- Sennewald, K.; Vogt, W.; Kandler, J.; Sommerfeld, R.; Sorbe, G. Process for Preparing Acrylonitrile. U.S. Patent Re27718, 7 August 1973. [Google Scholar]
- Yamaguchi, G.; Takenaka, S. Process for the Oxidation of Olefins to Aldehydes and Acids. U.S. Patent 3454630, 8 July 1969. [Google Scholar]
- Grasselli, R.K.; Miller, A.F.; Hardman, H.F. Catalysts and Process for the Ammoxidation of Olefins. U.S. Patent 4192776, 11 March 1980. [Google Scholar]
- Sugiyama, S.; Miyake, M.; Yoshida, M.; Nakao, Y.; Shimoda, N.; Katoh, M. Effect of cerium addition to bismuth-molybdenum complex oxide catalyst for partial oxidation of propylene to acrolein. J. Jpn. Pet. Inst. 2020, 63, 267–273. [Google Scholar] [CrossRef]
- Yang, H.; Fan, Y.; Wu, J.; Chen, Y. Structure and properties of BiCeVMoO mixed metal oxides catalysts for selective oxidation of propane. J. Mol. Catal. A: Chem. 2005, 227, 279–283. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, H.-L.; Weng, W.-Z.; Yi, X.-D. Selective Oxidation of Propane to Acrolein over Ce-Doped Ag-Mo-P-O Catalysts: Influence of Ce Promoter. Catal. Lett. 2003, 87, 229–234. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, H.; Weng, W. Reaction pathways for selective oxidation of propane to acrolein over Ce-Ag-Mo-P-O catalysts. Appl. Catal. A Gen. 2009, 353, 24–31. [Google Scholar] [CrossRef]
- Fischer, A.; Burkhardt, W.; Weckbecker, C.; Huthmacher, K.; Wilz, F. Mixed Oxide Catalysts for the Catalytic Gas-Phase Oxidation of Olefins. WO2007042369, 19 April 2007. [Google Scholar]
- Brazdil, J.F. Acrylonitrile. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Idol, J.D., Jr. Acrylonitrile. U.S. Patent 2904580, 15 September 1959. [Google Scholar]
- Veatch, F.; Callahan, J.L.; Milberger, E.C.; Foreman, R.W. Catalytic Oxidation of Propylene to Acrolein. In Proceedings of the 2nd International Congress on Catalysis, Paris, France, 4–8 July 1960; Volume 2, p. 2647. [Google Scholar]
- Giordano, N.; Bart, J.C.J. Structure-activity relations in cerium molybdenum tellurium oxide acrylonitrile catalysts. Geterog. Katal. 1979, 4 Pt 2, 27–32. [Google Scholar]
- Bart, J.C.J.; Giordano, N. Structure of the cerium-molybdenum-tellurium oxide acrylonitrile catalyst. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 56–62. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Giordano, N. Structure and activity of tellurium-molybdenum oxide acrylonitrile catalysts. J. Catal. 1980, 64, 356. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Giordano, N. Structure and activity of tellurium-cerium oxide acrylonitrile catalysts. J. Catal. 1982, 75, 134–139. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Giordano, N.; Forzatti, P. Phase relationships in the cerium-molybdenum-tellurium oxide system. ACS Symp. Ser. 1985, 279, 89–101. [Google Scholar] [CrossRef]
- Giordano, N.; Bart, J.C.J. Mechanism of ammoxidation of propene over cerium-doped bismuth molybdate catalysts. Recl. Trav. Chim. Pays-Bas 1975, 94, 28–30. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Grasselli, R.K. Relationship between solid state structure and catalytic activity of rare earth and bismuth-containing molybdate ammoxidation catalysts. J. Catal. 1983, 79, 104–117. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Glaeser, L.C.; Grasselli, R.K. Role of interfacial phenomena in the catalytic behavior of multiphase selective ammoxidation catalysts. J. Phys. Chem. 1983, 87, 5485–5491. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J. Catalytic properties of Ce containing composite oxide for ammoxidation of propylene. In Xinshiji De Cuihau Kexue Yu Jishu, Quanguo Cuihuaxue Jihuiyi Lunwenji, 10th, Zhangjiajie, China, 15–19 October 2000; Shanxi Kexue Jishu Chubanshe: Taiyuan, China, 2000; p. 245. [Google Scholar]
- Xu, H.; Liu, J. Catalytic behavior of cerium-containing multicomponent oxide for ammoxidation of propylene. Huaxue Shijie 2002, 43, 401–403. [Google Scholar]
- Aoki, K.; Honda, M.; Dozono, T.; Katsumata, T. Catalyst Composition. U.S. Patent 4443556, 17 April 1984. [Google Scholar]
- Suresh, D.D.; Friedrich, M.S.; Seely, M.J. Catalyst for Process for the Manufacture of Acrylonitrile and Methacrylonitrile. U.S. Patent 5093299, 3 March 1992. [Google Scholar]
- Suresh, D.D.; Friedrich, M.S.; Seely, M.J. Catalyst for the Manufacture of Acrylonitrile and Methacrylonitrile. U.S. Patent 5212137, 18 May 1993. [Google Scholar]
- Midorikawa, H.; Someya, K.; Aoki, K.; Nagano, O. Ammoxidation Catalyst Composition, and Process for Producing Acrylonitrile or Methacrylonitrile Using the Same. U.S. Patent 5658842, 19 August 1997. [Google Scholar]
- Paparizos, C.; Jevne, S.C.; Seely, M.J. Catalyst for the Manufacture of Acrylonitrile. U.S. Patent 7071140, 4 July 2006. [Google Scholar]
- Paparizos, C.; Jevne, S.C.; Seely, M.J. Catalyst for the Manufacture of Acrylonitrile. U.S. Patent 7348291, 25 March 2008. [Google Scholar]
- Zheng, Y.; Yang, T.; Yu, Z.; Zhao, J.; Cai, H.; Zhao, Q. Structure of active phases of cerium-containing multicomponent oxide and its catalytic behavior for ammoxidation of propylene. Yingyong Huaxue 1988, 5, 13–17. [Google Scholar]
- Brazdil, J.F.; Suresh, D.D.; Grasselli, R.K. Preparation of Acrylonitrile from Propylene, Oxygen, and Ammonia in the Presence of an Alkali Metal Promoted Bismuth, Cerium, Molybdenum, Tungsten Catalyst. U.S. Patent 4746753, 24 May 1988. [Google Scholar]
- Brazdil, J.F.; Lin, S.Y. Ammoxidation Catalysts Containing Samarium. U.S. Patent Application 20170114007, 27 April 2017. [Google Scholar]
- Yoshida, J.; Yamaguchi, T. Oxide Catalyst and Method for Producing the Same, and Methods for Producing Unsaturated Aldehyde, Diolefin, and Unsaturated Nitrile. U.S. Patent 9364817, 14 June 2016. [Google Scholar]
- Brazdil, J.F. Designing multifunctionality into single phase and multiphase metal-oxide-selective propylene ammoxidation catalysts. Catalysts 2018, 8, 103. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Toft, M.A.; McKenna, S.T. Mixed Metal Oxide Catalysts. U.S. Patent 9433929, 6 September 2016. [Google Scholar]
- Brazdil, J.F.; Toft, M.A. Mixed Metal Oxide Ammoxidation Catalysts. U.S. Patent Application 20160175817, 23 June 2016. [Google Scholar]
- Fukuzawa, A.; Kaneta, M. Fluid Bed Ammoxidation Reaction Catalyst Comprising Silica and Metal Oxide, and Production Method of Acrylonitrile Using Same. WO2017130906, 3 August 2017. [Google Scholar]
- Iitsuka, C.; Fukuzawa, A. Catalyst, Method for Manufacturing Catalyst, and Method for Manufacturing Acrylonitrile. WO2020213362, 22 October 2020. [Google Scholar]
- Ushikubo, T.; Oshima, K.; Kayo, A.; Umezawa, T.; Kiyono, K.-I.; Sawaki, I.; Nakamura, H. Molybdenum Oxide Ammoxidation Catalyst for the Production of Nitriles. U.S. Patent 5472925, 5 December 1995. [Google Scholar]
- Wang, G.; Guo, Y.; Lu, G. Promotional effect of cerium on Mo-V-Te-Nb mixed oxide catalyst for ammoxidation of propane to acrylonitrile. Fuel Process. Technol. 2015, 130, 71–77. [Google Scholar] [CrossRef]
- Grasselli, R.K. Selectivity issues in (amm)oxidation catalysis. Catal. Today 2005, 99, 23–31. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Buttrey, D.J.; Burrington, J.D.; Andersson, A.; Holmberg, J.; Ueda, W.; Kubo, J.; Lugmair, C.G.; Volpe, A.F. Active centers, catalytic behavior, symbiosis and redox properties of MoV(Nb,Ta)TeO ammoxidation catalysts. Top. Catal. 2006, 38, 7–16. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Buttrey, D.J.; DeSanto, P.; Burrington, J.D.; Lugmair, C.G.; Volpe, A.F.; Weingand, T. Active centers in Mo–V–Nb–Te–Ox (amm)oxidation catalysts. Catal. Today 2004, 91–92, 251–258. [Google Scholar] [CrossRef]
- Goddard, W.A., III; Mueller, J.E.; Chenoweth, K.; van Duin, A.C.T. ReaxFF Monte Carlo reactive dynamics: Application to resolving the partial occupations of the M1 phase of the MoVNbTeO catalyst. Catal. Today 2010, 157, 71–76. [Google Scholar] [CrossRef]
- Cheng, M.-J.; Goddard, W.A., III. In Silico Design of Highly Selective Mo-V-Te-Nb-O Mixed Metal Oxide Catalysts for Ammoxidation and Oxidative Dehydrogenation of Propane and Ethane. J. Am. Chem. Soc. 2015, 137, 13224–13227. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Kaneko, K. Method for Purification of Acrylonitrile Manufactured by Catalytic Ammoxidation of Propane. JP2010222309, 10 July 2010. [Google Scholar]
- Grand Opening Ceremony for AN and MMA Plants in Thailand. 2013. Available online: https://www.asahi-kasei.co.jp/asahi/en/news/2012/e130213_1.html (accessed on 18 May 2022).
- Ishii, Y.; Okada, K. Oxide Catalyst, Production Method Therefor, and Unsaturated-Nitrile Production Method. WO2015133510, 11 September 2015. [Google Scholar]
- Brazdil, J.F. The legacy and promise of heterogeneous selective oxidation and ammoxidation catalysis. Catal. Today 2021, 363, 55–59. [Google Scholar] [CrossRef]
- Bej, S.K.; Rao, M.S. Selective oxidation of n-butane to maleic anhydride. 1. Optimization studies. Ind. Eng. Chem. Res. 1991, 30, 1819–1824. [Google Scholar] [CrossRef]
- Bej, S.K.; Rao, M.S. Selective oxidation of n-butane to maleic anhydride: A comparative study between promoted and unpromoted VPO catalysts. Appl. Catal. A Gen. 1992, 83, 149–163. [Google Scholar] [CrossRef]
- López Nieto, J.M.; Solsona, B.; Védrine, J.C. 5-Gas phase heterogeneous partial oxidation reactions. In Metal Oxides in Heterogeneous Catalysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–286. [Google Scholar]
- Brazdil, J.F. Selective oxidation in industry: Applications of metal oxides in the petrochemical industry. In Metal Oxides in Heterogeneous Catalysis; Elsevier, B.V.: Amsterdam, The Netherlands, 2018; pp. 455–502. [Google Scholar]
- Daniell, W.; Ponchel, A.; Kuba, S.; Anderle, F.; Weingand, T.; Gregory, D.H.; Knoezinger, H. Characterization and Catalytic Behavior of VOx-CeO2 Catalysts for the Oxidative Dehydrogenation of Propane. Top. Catal. 2002, 20, 65–74. [Google Scholar] [CrossRef]
- Yun, Y.S.; Lee, M.; Sung, J.; Yun, D.; Kim, T.Y.; Park, H.; Lee, K.R.; Song, C.K.; Kim, Y.; Lee, J.; et al. Promoting effect of cerium on MoVTeNb mixed oxide catalyst for oxidative dehydrogenation of ethane to ethylene. Appl. Catal. B 2018, 237, 554–562. [Google Scholar] [CrossRef]
- Wan, C.; Cheng, D.-G.; Chen, F.; Zhan, X. The role of active phase in Ce modified BiMo catalysts for oxidative dehydrogenation of 1-butene. Catal. Today 2016, 264, 180–184. [Google Scholar] [CrossRef]
- Martinez-Huerta, M.V.; Coronado, J.M.; Fernandez-Garcia, M.; Iglesias-Juez, A.; Deo, G.; Fierro, J.L.G.; Banares, M.A. Nature of the vanadia-ceria interface in V5+/CeO2 catalysts and its relevance for the solid-state reaction toward CeVO4 and catalytic properties. J. Catal. 2004, 225, 240–248. [Google Scholar] [CrossRef]
- Park, S.M.; Jeon, S.W.; Kim, S.H. Formaldehyde Oxidation Over Manganese-Cerium-Aluminum Mixed Oxides Supported on Cordierite Monoliths at Low Temperatures. Catal. Lett. 2014, 144, 756–766. [Google Scholar] [CrossRef]
- Feng, T.; Vohs, J.M. A TPD study of the partial oxidation of methanol to formaldehyde on CeO2-supported vanadium oxide. J. Catal. 2004, 221, 619–629. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V.; Popa, C.; Sauer, J.; Abbott, H.; Uhl, A.; Baron, M.; Stacchiola, D.; Bondarchuk, O.; Shaikhutdinov, S.; Freund, H.-J. Role of Ceria in Oxidative Dehydrogenation on Supported Vanadia Catalysts. J. Am. Chem. Soc. 2010, 132, 2345–2349. [Google Scholar] [CrossRef]
- Kropp, T.; Paier, J.; Sauer, J. Support Effect in Oxide Catalysis: Methanol Oxidation on Vanadia/Ceria. J. Am. Chem. Soc. 2014, 136, 14616–14625. [Google Scholar] [CrossRef] [PubMed]
- Abbott, H.L.; Uhl, A.; Baron, M.; Lei, Y.; Meyer, R.J.; Stacchiola, D.J.; Bondarchuk, O.; Shaikhutdinov, S.; Freund, H.J. Relating methanol oxidation to the structure of ceria-supported vanadia monolayer catalysts. J. Catal. 2010, 272, 82–91. [Google Scholar] [CrossRef]
- Vining, W.C.; Strunk, J.; Bell, A.T. Investigation of the structure and activity of VOx/CeO2/SiO2 catalysts for methanol oxidation to formaldehyde. J. Catal. 2012, 285, 160–167. [Google Scholar] [CrossRef]
- Kropp, T.; Paier, J. Activity versus Selectivity of the Methanol Oxidation at Ceria Surfaces: A Comparative First-Principles Study. J. Phys. Chem. C 2015, 119, 23021–23031. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Bosco, M.V.; Bonivardi, A.; Busnengo, H.F.; Ganduglia-Pirovano, M.V. Insights into the Nature of Formate Species in the Decomposition and Reaction of Methanol over Cerium Oxide Surfaces: A Combined Infrared Spectroscopy and Density Functional Theory Study. J. Phys. Chem. C 2015, 119, 21452–21464. [Google Scholar] [CrossRef]
- Sutton, J.E.; Danielson, T.; Beste, A.; Savara, A. Below-Room-Temperature C–H Bond Breaking on an Inexpensive Metal Oxide: Methanol to Formaldehyde on CeO2(111). J. Phys. Chem. Lett. 2017, 8, 5810–5814. [Google Scholar] [CrossRef]
- Capdevila-Cortada, M.; García-Melchor, M.; López, N. Unraveling the structure sensitivity in methanol conversion on CeO2: A DFT+U study. J. Catal. 2015, 327, 58–64. [Google Scholar] [CrossRef]
- Brazdil, J.F., Jr.; Attig, T.G.; Grasselli, R.K. Ammoxidation of Methanol to Produce Hydrogen Cyanide. U.S. Patent 4485079, 27 November 1984. [Google Scholar]
- Haber, J. Selective oxidations: Fundamentals of hydrocarbon oxidation. In Handbook of Heterogeneous Catalysis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; p. 3359. [Google Scholar]
- Beckers, J.; Rothenberg, G. Sustainable selective oxidations using ceria-based materials. Green Chem. 2010, 12, 939–948. [Google Scholar] [CrossRef]
- Arena, F. Multipurpose composite MnCeOx catalysts for environmental applications. Catal. Sci. Technol. 2014, 4, 1890–1898. [Google Scholar] [CrossRef]
- Yoshida, H.; Koide, T.; Uemura, T.; Kuzuhara, Y.; Ohyama, J.; Machida, M. Ce-modified Rh Overlayer for a Three-Way Catalytic Converter with Oxygen Storage/Release Capability. Catal. Today, 2022, in press. [CrossRef]
- Wang, H.; Luo, S.; Zhang, M.; Liu, W.; Wu, X.; Liu, S. Roles of oxygen vacancy and Ox− in oxidation reactions over CeO2 and Ag/CeO2 nanorod model catalysts. J. Catal. 2018, 368, 365–378. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez Xavier Isidro, P.; DeLaRiva, A.; Wang, M.; Engelhard Mark, H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef]
- Salaev, M.A.; Salaeva, A.A.; Kharlamova, T.S.; Mamontov, G.V. Pt–CeO2-based composites in environmental catalysis: A review. Appl. Catal. B Environ. 2021, 295, 120286. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Wang, F. Room temperature HCHO oxidation over the Pt/CeO2 catalysts with different oxygen mobilities by changing ceria shapes. Appl. Catal. A Gen. 2022, 630, 118469. [Google Scholar] [CrossRef]
- Gaálová, J.; Topka, P. Gold and Ceria as Catalysts for VOC Abatement: A Review. Catalysts 2021, 11, 789. [Google Scholar] [CrossRef]
- Huang, Q.; Lu, Y.; Si, H.; Yang, B.; Tao, T.; Zhao, Y.; Chen, M. Study of Complete Oxidation of Formaldehyde Over MnOx–CeO2 Mixed Oxide Catalysts at Ambient Temperature. Catal. Lett. 2018, 148, 2880–2890. [Google Scholar] [CrossRef]
- Guan, S.; Huang, Q.; Ma, J.; Li, W.; Ogunbiyi, A.T.; Zhou, Z.; Chen, K.; Zhang, Q. HCHO Removal by MnO2(x)–CeO2: Influence of the Synergistic Effect on the Catalytic Activity. Ind. Eng. Chem. Res. 2020, 59, 596–608. [Google Scholar] [CrossRef]
- Davo-Quinonero, A.; Bailon-Garcia, E.; Lopez-Rodriguez, S.; Juan-Juan, J.; Lozano-Castello, D.; Garcia-Melchor, M.; Herrera, F.C.; Pellegrin, E.; Escudero, C.; Bueno-Lopez, A. Insights into the Oxygen Vacancy Filling Mechanism in CuO/CeO2 Catalysts: A Key Step Toward High Selectivity in Preferential CO Oxidation. ACS Catal. 2020, 10, 6532–6545. [Google Scholar] [CrossRef]
- Li, F.; Zhao, B.; Tan, Y.; Chen, W.; Tian, M. Preparation of Al2O3–CeO2 by Hydrothermal Method Supporting Copper Oxide for the Catalytic Oxidation of CO and C3H8. Ind. Eng. Chem. Res. 2022, 61, 4739–4751. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, K.; Xiong, J.; Ren, Q.; Zhong, J.; Cai, H.; Huang, H.; Chen, P.; Wu, J.; Chen, L.; et al. Static and Dynamic Quantification Tracking of Asymmetric Oxygen Vacancies in Copper-Ceria Catalysts with Superior Catalytic Activity. Appl. Catal. B Environ. 2022, 316, 121620. [Google Scholar] [CrossRef]
- Sun, P.; Wang, W.; Dai, X.; Weng, X.; Wu, Z. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1−xO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- Dong, J.; Li, D.; Zhang, Y.; Chang, P.; Jin, Q. Insights into the CeO2 facet-depended performance of propane oxidation over Pt-CeO2 catalysts. J. Catal. 2022, 407, 174–185. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Guan, X.; Wang, H.; Bao, H.; Huang, Y.; Qiao, J.; Zhou, G. Ruthenium nanoparticles supported on CeO2 for catalytic permanganate oxidation of butylparaben. Environ. Sci. Technol. 2013, 47, 13011–13019. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, K.; Zhang, X.; Tong, Q.; Ji, J.; Cai, Y.; Gao, B.; Zou, W.; Dong, L. CeO2 nanosheets with anion-induced oxygen vacancies for promoting photocatalytic toluene mineralization: Toluene adsorption and reactive oxygen species. Appl. Catal. B Environ. 2022, 317, 121694. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, B. Catalytic peroxidation of acrylonitrile aqueous solution by Ni-doped CeO2 catalysts: Characterization, kinetics and thermodynamics. Int. J. Environ. Sci. Technol. 2020, 17, 1809–1824. [Google Scholar] [CrossRef]
- Wei, X.; Wang, S.; Ni, C.; Wang, M.; Wang, S. Trade-off between redox ability and reactive behaviors for acrylonitrile selective catalytic combustion over the Cu-Ce-based UZM-9 catalysts. Appl. Catal. A Gen. 2021, 610, 117960. [Google Scholar] [CrossRef]
- Yi, Z.; Sun, J.; Li, J.; Yang, Y.; Zhou, T.; Wei, S.; Zhu, A. The highly efficient removal of HCN over Cu8Mn2/CeO2 catalytic material. RSC Adv. 2021, 11, 8886–8896. [Google Scholar] [CrossRef]
- Wen, M.; Dong, F.; Yao, J.; Tang, Z.; Zhang, J. Pt nanoparticles confined in the ordered mesoporous CeO2 as a highly efficient catalyst for the elimination of VOCs. J. Catal. 2022, 412, 42–58. [Google Scholar] [CrossRef]
- Maurer, F.; Beck, A.; Jelic, J.; Wang, W.; Mangold, S.; Stehle, M.; Wang, D.; Dolcet, P.; Gänzler, A.M.; Kübel, C.; et al. Surface Noble Metal Concentration on Ceria as a Key Descriptor for Efficient Catalytic CO Oxidation. ACS Catal. 2022, 12, 2473–2486. [Google Scholar] [CrossRef]
- Wang, Q.; Yeung, K.L.; Banares, M.A. Ceria and its related materials for VOC catalytic combustion: A review. Catal. Today 2020, 356, 141–154. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A review on the catalytic combustion of soot in Diesel particulate filters for automotive applications: From powder catalysts to structured reactors. Appl. Catal. A 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Zheng, C.; Mao, D.; Xu, Z.; Zheng, S. Strong Ru-CeO2 interaction boosts catalytic activity and stability of Ru supported on CeO2 nanocube for soot oxidation. J. Catal. 2022, 411, 122–134. [Google Scholar] [CrossRef]
- Jampaiah, D.; Velisoju, V.K.; Devaiah, D.; Singh, M.; Mayes, E.L.H.; Coyle, V.E.; Reddy, B.M.; Bansal, V.; Bhargava, S.K. Flower-like Mn3O4/CeO2 microspheres as an efficient catalyst for diesel soot and CO oxidation: Synergistic effects for enhanced catalytic performance. Appl. Surf. Sci. 2019, 473, 209–221. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Pan, Z.; Feng, F.; Gu, Y.; Du, J.; Zhao, Y. Design of CeMnCu ternary mixed oxides as soot combustion catalysts based on optimized Ce/Mn and Mn/Cu ratios in binary mixed oxides. Appl. Catal. B Environ. 2020, 268, 118422. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Ran, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; Giménez-Mañogil, J.; Martínez-Munuera, J.C.; Bueno-López, A.; García-García, A. Study of Ce/Pr ratio in ceria-praseodymia catalysts for soot combustion under different atmospheres. Appl. Catal. A Gen. 2020, 590, 117339. [Google Scholar] [CrossRef]
- Fahed, S.; Pointecouteau, R.; Aouine, M.; Boréave, A.; Gil, S.; Meille, V.; Bazin, P.; Toulemonde, O.; Demourgues, A.; Daturi, M.; et al. Pr-rich Cerium-zirconium-praseodymium Mixed Oxides for Automotive Exhaust Emission Control. Appl. Catal. A Gen. 2022, 644, 118800. [Google Scholar] [CrossRef]
- Levasseur, B.; Kaliaguine, S. Effects of iron and cerium in La1−yCeyCo1−xFexO3 perovskites as catalysts for VOC oxidation. Appl. Catal. B Environ. 2009, 88, 305–314. [Google Scholar] [CrossRef]
- Zhang, J.; Nazarenko, Y.; Zhang, L.; Calderon, L.; Lee, K.-B.; Garfunkel, E.; Schwander, S.; Tetley, T.D.; Chung, K.F.; Porter, A.E.; et al. Impacts of a Nanosized Ceria Additive on Diesel Engine Emissions of Particulate and Gaseous Pollutants. Environ. Sci. Technol. 2013, 47, 13077–13085. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, G.; Wu, X.; Gardener, M.; Park, B.; Anderson, S. Envirox™ fuel-borne catalyst: Developing and launching a nano-fuel additive. Technol. Anal. Strateg. Manag. 2008, 20, 127–136. [Google Scholar] [CrossRef]
- Cerion Energy’s Ground Breaking GO2 Diesel Fuel Additive Wins Most ECO-Friendly Product in the DAME Awards 2011. Available online: http://www.nanotech-now.com/news.cgi?story_id=43885 (accessed on 16 June 2022).
- Roos, J.W.; Richardson, D.; Claydon, D.J. Diesel Fuel Additives Containing Cerium or Manganese and Detergents. U.S. Patent Application 20080066375, 20 March 2008. [Google Scholar]
- Difrancesco, A.G.; Hailstone, R.K.; Langner, A.; Reed, K.J. Cerium Dioxide Nanoparticle Manufacture for Use as Combustion Improvers in Diesel Fuel and Biodiesel. WO2008030815, 13 March 2008. [Google Scholar]
- Lin, K.-S.; Chowdhury, S. Synthesis, characterization, and application of 1-D cerium oxide nanomaterials: A review. Int. J. Mol. Sci. 2010, 11, 3226–3251. [Google Scholar] [CrossRef] [PubMed]
- So, Y.-M.; Leung, W.-H. Recent advances in the coordination chemistry of cerium(IV) complexes. Coord. Chem. Rev. 2017, 340, 172–197. [Google Scholar] [CrossRef]
- Zhu, M.; Lai, J.-K.; Tumuluri, U.; Ford, M.E.; Wu, Z.; Wachs, I.E. Reaction Pathways and Kinetics for Selective Catalytic Reduction (SCR) of Acidic NOx Emissions from Power Plants with NH3. ACS Catal. 2017, 7, 8358–8361. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, C.; Dong, F.; Sun, X. Mechanistic insight into the selective catalytic reduction of NOx with propene on the Ce0.875Zr0.125O2 (110) surface. Catal. Sci. Technol. 2022, 12, 3685–3694. [Google Scholar] [CrossRef]
- Krocher, O. Selective Catalytic Reduction of NOx. Catalysts 2018, 8, 459. [Google Scholar] [CrossRef]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Lu, G.; Tang, Z. Recent Progress on Establishing Structure–Activity Relationship of Catalysts for Selective Catalytic Reduction (SCR) of NOx with NH3. Catal. Surv. Asia 2018, 22, 1–19. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, P.; Zhu, B. Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration. Materials 2018, 11, 1632. [Google Scholar] [CrossRef]
- Shan, W.; Liu, F.; Yu, Y.; He, H. The use of ceria for the selective catalytic reduction of NOx with NH3. Chin. J. Catal. 2014, 35, 1251–1259. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D.; Ran, R.; Yu, J. Rare earth containing catalysts for selective catalytic reduction of NOx with ammonia: A Review. J. Rare Earths 2014, 32, 907–917. [Google Scholar] [CrossRef]
- Hu, W.; Zou, R.; Dong, Y.; Zhang, S.; Song, H.; Liu, S.; Zheng, C.; Nova, I.; Tronconi, E.; Gao, X. Synergy of vanadia and ceria in the reaction mechanism of low-temperature selective catalytic reduction of NOx by NH3. J. Catal. 2020, 391, 145–154. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, J. Structure–activity relationship of VOx/CeO2 nanorod for NO removal with ammonia. Appl. Catal. B Environ. 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. Promotional Effect of Ce-doped V2O5-WO3/TiO2 with Low Vanadium Loadings for Selective Catalytic Reduction of NOx by NH3. J. Phys. Chem. C 2009, 113, 21177–21184. [Google Scholar] [CrossRef]
- Cai, M.; Bian, X.; Xie, F.; Wu, W.; Cen, P. Preparation and performance of cerium-based catalysts for selective catalytic reduction of nitrogen oxides: A critical review. Catalysts 2021, 11, 361. [Google Scholar] [CrossRef]
- Xu, J.; Yu, H.; Zhang, C.; Guo, F.; Xie, J. Development of cerium-based catalysts for selective catalytic reduction of nitrogen oxides: A review. New J. Chem. 2019, 43, 3996–4007. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Huang, Z.; Shen, H.; Jing, G. MnOx-decorated VOx/CeO2 catalysts with preferentially exposed {110} facets for selective catalytic reduction of NOx by NH3. Appl. Catal. B Environ. 2020, 268, 118419. [Google Scholar] [CrossRef]
- Sun, H.; Park, S.-J. Recent Advances in MnOx/CeO2-Based Ternary Composites for Selective Catalytic Reduction of NOx by NH3: A Review. Catalysts 2021, 11, 1519. [Google Scholar] [CrossRef]
- Andana, T.; Rappé, K.G.; Nelson, N.C.; Gao, F.; Wang, Y. Selective catalytic reduction of NOx with NH3 over Ce-Mn oxide and Cu-SSZ-13 composite catalysts—Low temperature enhancement. Appl. Catal. B Environ. 2022, 316, 121522. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, A.; Zhang, J.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W. Low-Temperature NH3-SCR on Cex-Mn-Tiy Mixed Oxide Catalysts: Improved Performance by the Mutual Effect between Ce and Ti. Catalysts 2022, 12, 471. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, R.-T.; Zhang, X.-F.; Liu, Y.-Z.; Duan, C.-P.; Wu, G.-L.; Pan, W.-G. Cerium Oxide-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx with NH3: A Review. Energy Fuels 2021, 35, 2981–2998. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Dong, L. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 1248–1264. [Google Scholar] [CrossRef]
- Zhou, Q.; He, K.; Wang, X.; Lim, K.H.; Liu, P.; Wang, W.; Wang, Q. Investigation on the redox/acidic features of bimetallic MOF-derived CeMOx catalysts for low-temperature NH3-SCR of NOx. Appl. Catal. A Gen. 2022, 643, 118796. [Google Scholar] [CrossRef]
- Yang, S.; Kim, M.; Yang, S.; Kim, D.S.; Lee, W.J.; Lee, H. Production of acrylic acid from biomass-derived allyl alcohol by selective oxidation using Au/ceria catalysts. Catal. Sci. Technol. 2016, 6, 3616–3622. [Google Scholar] [CrossRef]
- Available online: https://cen.acs.org/articles/88/i21/Arkema-Pushes-Biobased-Acrylics.html (accessed on 25 August 2022).
- A Plant-Based Solution. Available online: https://www.adm.com/en-us/products-services/industrial-biosolutions/products/propylene-glycol (accessed on 6 July 2022).
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Smith, L.R.; Sainna, M.A.; Douthwaite, M.; Davies, T.E.; Dummer, N.F.; Willock, D.J.; Knight, D.W.; Catlow, C.R.A.; Taylor, S.H.; Hutchings, G.J. Gas Phase Glycerol Valorization over Ceria Nanostructures with Well-Defined Morphologies. ACS Catal. 2021, 11, 4893–4907. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Hutchings, G.J. Green Catalysis with Alternative Feedstocks. Top. Catal. 2009, 52, 258–268. [Google Scholar] [CrossRef]
- Miedziak, P.J.; Alshammari, H.; Kondrat, S.A.; Clarke, T.J.; Davies, T.E.; Morad, M.; Morgan, D.J.; Willock, D.J.; Knight, D.W.; Taylor, S.H.; et al. Base-free glucose oxidation using air with supported gold catalysts. Green Chem. 2014, 16, 3132–3141. [Google Scholar] [CrossRef]
- Albrecht, K.; Brazdil, J. Processes for Preparing Aldaric, Aldonic, and Uronic Acids. WO2021101810, 27 May 2021. [Google Scholar]
- Qi, X.; He, Y.; Yao, Y.; Li, Y.; Zhang, L.; Geng, M.; Wei, H.; Chu, H. Effect of CeO2 morphology on the catalytic properties of Au/CeO2 for base-free glucose oxidation. Catal. Sci. Technol. 2022, 12, 1313–1323. [Google Scholar] [CrossRef]
- Ventura, M.; Lobefaro, F.; de Giglio, E.; Distaso, M.; Nocito, F.; Dibenedetto, A. Selective Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran or 2-Formyl-5-furancarboxylic Acid in Water by using MgO·CeO2 Mixed Oxides as Catalysts. ChemSusChem 2018, 11, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, C.; Liu, X.; Xia, Q.; Wang, Y. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over MnOx–CeO2 composite catalysts. Green Chem. 2017, 19, 996–1004. [Google Scholar] [CrossRef]
- Gao, P.; Kang, Z.; Fu, W.; Wang, W.; Bai, X.; Wang, E. Electrically Driven Redox Process in Cerium Oxides. J. Am. Chem. Soc. 2010, 132, 4197–4201. [Google Scholar] [CrossRef]
- Yashima, M.; Hirose, T.; Katano, S.; Suzuki, Y.; Kakihana, M.; Yoshimura, M. Structural changes of ZrO2-CeO2 solid solutions around the monoclinic-tetragonal phase boundary. Phys. Rev. B Condens. Matter 1995, 51, 8018. [Google Scholar] [CrossRef] [PubMed]
- Van den Elzen, A.F.; Rieck, G.D. The crystal structure of Bi2(MoO4)3. Acta Crystallogr. Sect. B 1973, 29, 2433–2436. [Google Scholar] [CrossRef]
- Brixner, L.H.; Sleight, A.W.; Licis, M.S. Cell dimensions of the molybdates La2(MoO4)3, Ce2(MoO4)3, Pr2(MoO4)3, and Nd2(MoO4)3. J. Solid State Chem. 1972, 5, 247–249. [Google Scholar] [CrossRef]
- Brazdil, J.F. Scheelite: A versatile structural template for selective alkene oxidation catalysts. Catal. Sci. Technol. 2015, 5, 3452–3458. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Toft, M.A.; Lin, S.S.Y.; McKenna, S.T.; Zajac, G.; Kaduk, J.A.; Golab, J.T. Characterization of bismuth–cerium-molybdate selective propylene ammoxidation catalysts. Appl. Catal. A Gen. 2015, 495, 115–123. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber Ashleigh, E.; Evans, J.; Senanayake Sanjaya, D.; Stacchiola Darío, J.; Liu, P.; Hrbek, J.; Sanz Javier, F.; et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of Metal Nanocrystal Size Reveals Metal-Support Interface Role for Ceria Catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Zolotova, E.S.; Trushnikova, L.N.; Ayupov, B.M.; Sokolov, V.V.; Daletskii, V.A. Na2MoO4-CaMoO4-Ce2/3MoO4 scheelite-like solid solutions. Inorg. Mater. 2009, 45, 432–435. [Google Scholar] [CrossRef]
- Kruth, A.; Mather, G.C.; Jurado, J.R.; Irvine, J.T.S. Anomalous variations of unit cell parameters with composition in proton conducting, ACeO3-type perovskite solid solutions. Solid State Ion. 2005, 176, 703–712. [Google Scholar] [CrossRef]
- Varez, A.; Garcia-Gonzalez, E.; Sanz, J. Cation miscibility in CeO2-ZrO2 oxides with fluorite structure. A combined TEM, SAED and XRD Rietveld analysis. J. Mater. Chem. 2006, 16, 4249–4256. [Google Scholar] [CrossRef]
- Tian, F. A Review of Solid-Solution Models of High-Entropy Alloys Based on Ab Initio Calculations. Front. Mater. 2017, 4, 36. [Google Scholar] [CrossRef]
- Stenzel, D.; Issac, I.; Wang, K.; Azmi, R.; Singh, R.; Jeong, J.; Najib, S.; Bhattacharya, S.S.; Hahn, H.; Brezesinski, T.; et al. High Entropy and Low Symmetry: Triclinic High-Entropy Molybdates. Inorg. Chem. 2021, 60, 115–123. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Yu, H.; Zou, D. High-entropy oxides for catalysis: Status and perspectives. Appl. Catal. A Gen. 2022, 631, 118478. [Google Scholar] [CrossRef]
- Zheng, Z.; Ji, H.; Zhang, Y.; Cai, J.; Mo, C. High-entropy (Ca0.5Ce0.5)(Nb0.25Ta0.25Mo0.25W0.25)O4 scheelite ceramics with high-temperature negative temperature coefficient (NTC) property for thermistor materials. Solid State Ion. 2022, 377, 115872. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, H.; Huang, Y.; Luo, X.; Mao, Y.; Liang, Z. Study of Direct Synthesis of DMC from CO2 and Methanol on CeO2: Theoretical Calculation and Experiment. Ind. Eng. Chem. Res. 2022. [Google Scholar] [CrossRef]
- Ogada, J.J.; Ipadeola, A.K.; Mwonga, P.V.; Haruna, A.B.; Nichols, F.; Chen, S.; Miller, H.A.; Pagliaro, M.V.; Vizza, F.; Varcoe, J.R.; et al. CeO2 Modulates the Electronic States of a Palladium Onion-Like Carbon Interface into a Highly Active and Durable Electrocatalyst for Hydrogen Oxidation in Anion-Exchange-Membrane Fuel Cells. ACS Catal. 2022, 12, 7014–7029. [Google Scholar] [CrossRef]
- Ji, G.; Zhao, L.; Wang, Y.; Tang, Y.; He, C.; Liu, S.; Duan, C. A Binuclear Cerium-Based Metal–Organic Framework as an Artificial Monooxygenase for the Saturated Hydrocarbon Aerobic Oxidation with High Efficiency and High Selectivity. ACS Catal. 2022, 12, 7821–7832. [Google Scholar] [CrossRef]
- Zoller, S.; Koepf, E.; Nizamian, D.; Stephan, M.; Patané, A.; Haueter, P.; Romero, M.; González-Aguilar, J.; Lieftink, D.; de Wit, E.; et al. A solar tower fuel plant for the thermochemical production of kerosene from H2O and CO2. Joule 2022, 6, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Solar-Powered Reactor Converts CO2 and Water into Jet Fuel. Available online: https://cendigitalmagazine.acs.org/2022/08/01/solar-powered-reactor-converts-co%E2%82%82-and-water-into-jet-fuel/content.html (accessed on 3 August 2022).







| Wt.% Ce in BiMoOxide | % Propene Conversion | % Acrolein Selectivity | % Acrolein Yield |
|---|---|---|---|
| 0 | 81.6 | 70.7 | 57.7 |
| 1 | 78.2 | 72.1 | 56.4 |
| 3 | 78.6 | 73.8 | 58.0 |
| 5 | 82.7 | 73.5 | 60.8 |
| 10 | 86.0 | 75.7 | 65.1 |
| Catalyst Composition | Reaction Temperature (°C) | % Propene Conversion | % Acrylonitrile Yield |
|---|---|---|---|
| Bi4Ce4Mo12Ox | 430 | 100 | 65.8 |
| 460 | 100 | 71.5 | |
| Bi4Ce4Mo10W2Ox | 430 | 98.6 | 69.5 |
| K0.1Bi4Ce4Mo12Ox | 460 | 99.3 | 79.8 |
| Cs0.02Bi4Ce4Mo12Ox | 445 | 98.4 | 74.4 |
| Cs0.04Bi4Ce4Mo8W4Ox | 460 | 99.5 | 81.8 |
| Cs0.04Bi4Ce4SbMo10W2Ox | 460 | 97.9 | 80.0 |
| Elements | % Acrylonitrile Yield | |||||
|---|---|---|---|---|---|---|
| Cs | Co | Fe | Bi | Mo | 76.3 [47] | |
| Cs | Co | Fe | Bi | Ce | Mo | 86.4 [47] |
| Rb | Co Ni Mg | Fe | Bi | Ce | Mo | 86.0 [47] |
| Cs | Co | Fe | Bi | Pr | Mo | 85.7 [47] |
| Rb | Ni Mg | Fe Cr | Bi | Ce Sm | Mo | 85.8 [46] |
| Catalyst Composition | Reaction Temperature (°C) | % HCN Yield |
|---|---|---|
| K0.1Bi4Ce4Mo12Ox | 410 | 75.2 |
| K0.1Bi4La4Mo12Ox | 410 | 55.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazdil, J.F. The Emergence of the Ubiquity of Cerium in Heterogeneous Oxidation Catalysis Science and Technology. Catalysts 2022, 12, 959. https://doi.org/10.3390/catal12090959
Brazdil JF. The Emergence of the Ubiquity of Cerium in Heterogeneous Oxidation Catalysis Science and Technology. Catalysts. 2022; 12(9):959. https://doi.org/10.3390/catal12090959
Chicago/Turabian StyleBrazdil, James F. 2022. "The Emergence of the Ubiquity of Cerium in Heterogeneous Oxidation Catalysis Science and Technology" Catalysts 12, no. 9: 959. https://doi.org/10.3390/catal12090959
APA StyleBrazdil, J. F. (2022). The Emergence of the Ubiquity of Cerium in Heterogeneous Oxidation Catalysis Science and Technology. Catalysts, 12(9), 959. https://doi.org/10.3390/catal12090959