Effective Production of 5-Hydroxymethylfurfural from Fructose over a Highly Active Sulfonic Acid Functionalized SBA-15 Catalyst
Abstract
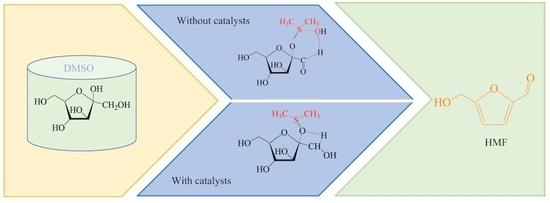
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Sulfonic-Acid-Functionalized SBA-15 Catalyst
2.3. Catalyst Characterization
2.4. Catalytic Performance
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Catalyst Activity
3.3. Comparison of the Activity with Other Reported Catalysts
3.4. Kinetic Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Qiu, G.; Huang, C.; Sun, X.; Chen, B. Highly active niobium-loaded montmorillonite catalysts for the production of 5-hydroxymethylfurfural from glucose. Green Chem. 2019, 21, 3930–3939. [Google Scholar] [CrossRef]
- Nunes, R.S.; Reis, G.M.; Vieira, L.M.; Mandelli, D.; Carvalho, W.A. Ultra-fast selective fructose dehydration promoted by a kraft lignin sulfonated carbon under microwave heating. Catal. Lett. 2021, 151, 398–408. [Google Scholar] [CrossRef]
- Antal Jr, M.J.; Mok, W.S.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohyd. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Production of hydroxymethylfurfural from fructose in a biphasic system using aqueous- and organic- phase modifiers. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Acid-catalyzed production of 5-hydroxymethyl furfural from D-fructose in subcritical water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Qi, X.H.; Guo, H.X.; Li, L.Y. Efficient conversion of fructose to 5-hydroxymethylfurfural catalyzed by sulfated zirconia in ionic liquids. Ind. Eng. Chem. Res. 2011, 50, 7985–7989. [Google Scholar] [CrossRef]
- Qi, X.H.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Efficient catalytic conversion of fructose into 5-hydroxymethylfurfural in ionic liquids at room temperature. Chem. Sustain. Energy Mater. 2009, 2, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, L.J.; Yang, J.R.; Wang, X.Q.; Qin, X.Y.; Qi, X.H.; Shen, F. Eco-friendly synthesis of SO3H-containing solid acid via mechanochemistry for the conversion of carbohydrates to 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2020, 8, 7059–7067. [Google Scholar] [CrossRef]
- Gomes, F.; Mendes, F.; Souza, M. Synthesis of 5-hydroxymethylfurfural from fructose catalyzed by phosphotungstic acid. Catal. Today 2017, 279, 296–304. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Du, M.; Ji, P.J. Multifunctional tin-based heterogeneous catalyst for catalytic conversion of glucose to 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2018, 6, 5636–5644. [Google Scholar] [CrossRef]
- Chen, G.Z.; Sun, Q.H.; Xu, J.; Zheng, L.F.; Rong, J.F.; Zong, B.N. Sulfonic Derivatives as Recyclable Acid Catalysts in the Dehydration of Fructose to 5-Hydroxymethylfurfural in Biphasic Solvent Systems. ACS Omega 2021, 6, 6798–6809. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Melero, J.A.; Paniagua, M.; Iglesias, J.; Hernández, B.; Sanz, M. Sulfonic acid heterogeneous catalysts for dehydration of C6-monosaccharides to 5-hydroxymethylfurfural in dimethyl sulfoxide. Chin. J. Catal. 2014, 35, 644–655. [Google Scholar] [CrossRef]
- Wei, W.Q.; Lyu, G.J.; Jiang, W.K.; Chen, Z.Y.; Wu, S.B. High-efficiency synthesis of 5-hydroxymethylfurfural and 2, 5-diformylfuran from fructose over magnetic separable catalysts. J. Colloid Interf. Sci. 2021, 602, 146–158. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Xu, W.; Yu, B.; Zhang, Y.; Chen, X.; Zhang, G.F.; Gao, Z.W. Single-site SBA-15 supported zirconium catalysts. Synthesis, characterization and toward cyanosilylation reaction. Appl. Surf. Sci. 2015, 325, 227–234. [Google Scholar] [CrossRef]
- Zhang, J.H.; Cheng, K.; Li, H.Y.; Yin, F.W.; Wang, Q.; Cui, L.; Yang, S.S.; Nie, J.G.; Zhou, D.Y.; Zhu, B.W. Efficient Synthesis of Structured Phospholipids Containing Short-Chain Fatty Acids over a Sulfonated Zn-SBA-15 Catalyst. J. Agric. Food Chem. 2020, 8, 12444–12453. [Google Scholar] [CrossRef]
- Héroguel, F.; Silvioli, L.; Du, Y.P.; Luterbacher, J.S. Controlled deposition of titanium oxide overcoats by non-hydrolytic sol gel for improved catalyst selectivity and stability. J. Catal. 2018, 358, 50–61. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Pulingam, T.; Rajabathar, J.R.; Khoerunnisa, F.; Ling, T.C.; Tan, S.h.; Ng, E. Acid-base bifunctional SBA-15 as an active and selective catalyst for synthesis of ethyl α-cyanocinnamate via Knoevenagel condensation. Microporous Mesoporous Mater. 2021, 320, 111091–111101. [Google Scholar] [CrossRef]
- Joseph, T.; Deshpande, S.S.; Halligudi, S.B.; Vinu, A.; Ernst, S.; Hartmann, M. Hydrogenation of olefins over hydrido chlorocarbonyl tris-(triphenylphosphine) ruthenium (II) complex immobilized on functionalized MCM-41 and SBA-15. J. Mol. Catal. A-Chem. 2003, 206, 13–21. [Google Scholar] [CrossRef]
- Suib, S.L.; Přech, J.; Čejka, J.; Kuwahara, Y.; Mori, K.; Yamashita, H. Some novel porous materials for selective catalytic oxidations. Mater. Today 2020, 32, 244–259. [Google Scholar] [CrossRef]
- Gatti, G.; Costenaro, D.; Vittoni, C.; Paul, G.; Crocellà, V.; Mangano, E.; Brandani, S.; Bordiga, S.; Cossi, M.; Marchese, L.; et al. CO2 adsorption on different organo-modified SBA-15 silicas: A multidisciplinary study on the effects of basic surface groups. Phys. Chem. Chem. Phys. 2017, 19, 14114–14128. [Google Scholar] [CrossRef] [PubMed]
- Vavsari, V.F.; Ziarani, G.M.; Badiei, A. The role of SBA-15 in drug delivery. RSC Adv. 2015, 5, 91686–91707. [Google Scholar] [CrossRef]
- Xiang, Y.P.; Wen, S.; Tian, Y.; Zhao, K.Y.; Guo, D.W.; Cheng, F.; Xu, Q.; Liu, X.X.; Yin, D.L. Efficient synthesis of 5-ethoxymethylfurfural from biomass-derived 5-hydroxymethylfurfural over sulfonated organic polymer catalyst. RSC Adv. 2021, 11, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Li, J.R.; Wang, K.K.; Han, T.T.; Tong, M.M.; Li, L.S.; Xie, Y.B.; Yang, Q.Y.; Liu, D.H.; Zhong, C.L. An in Situ Self-Assembly Template Strategy for The Preparation of Hierarchical-Pore Metal-Organic Frameworks. Nat. Commun. 2015, 6, 1–8. Available online: https://www.nature.com/articles/ncomms9847 (accessed on 28 August 2022). [CrossRef] [PubMed]
- Li, X.L.; Li, M.M.; Liu, Y.X.; Feng, Y.S.; Pan, P. Preparation of 5-hydroxymethylfurfural using magnetic Fe3O4@SiO2@mSiO2-TaOPO4 catalyst in 2-pentanol. Rsc. Adv. 2022, 12, 13251–13260. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.W.; Mao, W.; Ye, G.R.; Xia, Y.; Wei, C.; Zeng, L.; Zhou, J.H. Tin–chromium bimetallic metal–organic framework MIL-101 (Cr, Sn) as a catalyst for glucose conversion into HMF. Biomass Bioenergy 2022, 159, 106395–106404. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Gao, Z.; Wang, L.; Zhang, J. Leaf-derived sulfonated carbon dots: Efficient and recoverable catalysts to synthesize 5-hydroxymethylfurfural from fructose. Mater. Today Chem. 2021, 20, 100423–100430. [Google Scholar] [CrossRef]
- Jing, Y.X.; Guo, Y.; Xia, Q.; Liu, X.H.; Wang, Y.Q. Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellulosic Biomass. Chem 2019, 5, 1–27. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Wu, C.W. Conversion and kinetics study of fructose-to-5-hydroxymethylfurfural (HMF) using sulfonic and ionic liquid groups bi-functionalized mesoporous silica nanoparticles as recyclable solid catalysts in DMSO systems. Phys. Chem. Chem. Phys. 2012, 14, 13914–13917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.B.; Zhu, L.F.; Hu, C.W. High-efficiency synthesis of 5-hydroxymethylfurfural from fructose over highly sulfonated organocatalyst. Ind. Eng. Chem. Res. 2020, 59, 17218–17227. [Google Scholar] [CrossRef]
- Li, K.X.; Chen, J.; Yan, Y.B.; Min, Y.G.; Li, H.P.; Xi, F.N.; Liu, J.Y.; Chen, P. Quasi-homogeneous carbocatalysis for one-pot selective conversion of carbohydrates to 5-hydroxymethylfurfural using sulfonated graphene quantum dots. Carbon 2018, 136, 224–233. [Google Scholar] [CrossRef]
- Tong, X.L.; Li, Y.D. Efficient and selective dehydration of fructose to 5-hydroxymethylfurfural catalyzed by Brönsted-acidic ionic liquids. Chem. Sustain. Energy Mater. 2010, 3, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, G.S.; Kumar, R.; Wyman, C.E.; Christopher, P. Unifying mechanistic analysis of factors controlling selectivity in fructose dehydration to 5-hydroxymethylfurfural by homogeneous acid catalysts in aprotic solvents. ACS Catal. 2018, 8, 5591–5600. [Google Scholar] [CrossRef]
- Shi, S.B.; Wu, Y.F.; Liu, P.L.; Zhang, M.T.; Zhang, Z.Q.; Gao, L.J.; Xiao, G.M. Efficient conversion of carbohydrates to 5-hydroxymethylfurfural over poly(4-styrenesulfonic acid) catalyst. Catal. Lett. 2022, 152, 954–961. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.B.; Li, H.Y.; Ma, Y.B.; Zhang, R.H. High selective production of 5-hydroxymethylfurfural from fructose by sulfonic acid functionalized SBA-15 catalyst. Compos. Part B-Eng. 2019, 156, 88–94. [Google Scholar] [CrossRef]








| Catalyst | BET Surface Area (m2 g−1) 1 | Pore Volume (cm3 g−1) 1 | Pore Size (nm) 1 | S-Element Content (wt%) 2 | L (mmol Py·g−1) 3 | B (mmol Py·g−1) 3 |
|---|---|---|---|---|---|---|
| SBA-15 | 868 | 1.29 | 5.96 | / | / | / |
| SBA-15-SO3H | 200 | 0.48 | 9.66 | 9.08 | 0 | 0.34 |
| Entry | Catalysts | Reaction Condition | Catalyst Activity | Ref. | ||
|---|---|---|---|---|---|---|
| Temp (°C) | Time (h) | Yield (%) | Selectivity (%) | |||
| 1 | (HSO3 + CP)-MSN | 90 | 1 | 35 | / | [31] |
| 2 | S-A-PAN-H | 140 | 0.75 | 88.8 | 89 | [32] |
| 3 | SGQDs | 170 | 2 | 51.7 | 56.3 | [33] |
| 4 | [NMP]+[HSO4]- | 90 | 2 | 69.4 | 70.4 | [34] |
| 5 | H2SO4 | 150 | 0.5 | / | 84 | [35] |
| 6 | PSS | 130 | 6 | 48.5 | / | [36] |
| 7 | SBA-15-SO3H | 80 | 1 | 58.4 | / | This work |
| 8 | SBA-15-SO3H | 130 | 1 | 78.7 | / | This work |
| 9 | Without catalysts | 130 | 1 | 46.9 | / | This work |
| 10 | SBA-15 | 130 | 1 | 45.6 | / | This work |
| Temperature (°C) | K | Ea (kJ/mol) | R2 | |
|---|---|---|---|---|
| With catalysts | 60 | 0.018 | 56.99 | 0.9992 |
| 80 | 0.0548 | |||
| 100 | 0.1637 | |||
| Without catalysts | 60 | 0.0048 | 109.35 | 0.9017 |
| 80 | 0.0137 | |||
| 100 | 0.346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Song, K.; Xu, X.; He, J.; Guo, J. Effective Production of 5-Hydroxymethylfurfural from Fructose over a Highly Active Sulfonic Acid Functionalized SBA-15 Catalyst. Catalysts 2022, 12, 984. https://doi.org/10.3390/catal12090984
Zhu Y, Song K, Xu X, He J, Guo J. Effective Production of 5-Hydroxymethylfurfural from Fructose over a Highly Active Sulfonic Acid Functionalized SBA-15 Catalyst. Catalysts. 2022; 12(9):984. https://doi.org/10.3390/catal12090984
Chicago/Turabian StyleZhu, Yutong, Ke Song, Xiaofei Xu, Jian He, and Jie Guo. 2022. "Effective Production of 5-Hydroxymethylfurfural from Fructose over a Highly Active Sulfonic Acid Functionalized SBA-15 Catalyst" Catalysts 12, no. 9: 984. https://doi.org/10.3390/catal12090984
APA StyleZhu, Y., Song, K., Xu, X., He, J., & Guo, J. (2022). Effective Production of 5-Hydroxymethylfurfural from Fructose over a Highly Active Sulfonic Acid Functionalized SBA-15 Catalyst. Catalysts, 12(9), 984. https://doi.org/10.3390/catal12090984