Oxygen-Deficient Engineering for Perovskite Oxides in the Application of AOPs: Regulation, Detection, and Reduction Mechanism
Abstract
:1. Introduction
2. Perovskite Materials
2.1. Structure of Perovskite
2.2. The Role of Oxygen Vacancy in Perovskite


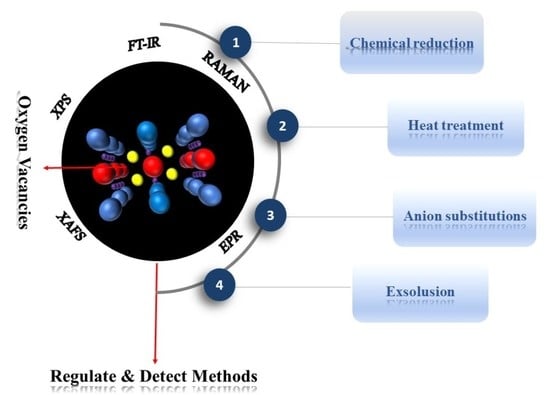
3. Approaches to Regulate Oxygen Vacancy in Perovskite
3.1. Ion Substitutions
3.2. Ion Doping
3.3. Heat Treatment
3.4. Wet-Chemical Redox Reactions
3.5. Exsolution
3.6. Etching Method
4. Technologies for Detecting Oxygen Vacancies
4.1. Spectral Detection Methods
4.1.1. X-ray Photoelectron Spectroscopy (XPS)
4.1.2. Raman Spectroscopy
4.1.3. The X-ray Absorption Microstructural Spectrum (XAFS)
4.2. Electron Paramagnetic Resonance
4.3. Kelvin Probe Force Microscope and Electron Microscopy
4.4. H2-TPR, O2-TPD
4.5. Gravimetric Method
5. Reduction Mechanism of Oxygen Vacancies
5.1. Roles of Oxygen Vacancies in the ROS
5.2. Evaluation of the Catalytic Activity of Oxygen Vacancies
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urán, L.; Gallego, J.; Li, W.-Y.; Santamaría, A. Effect of catalyst preparation for the simultaneous removal of soot and NOx. Appl. Catal. A 2019, 569, 157–169. [Google Scholar] [CrossRef]
- Liu, N.; Dang, Y.; Hu, B.; Tian, M.; Jiang, H.; Quan, G.; Qiao, R.; Lei, J.; Zhang, X. BN/Fe3O4/MIL-53(Fe) ternary nanocomposite for boosted ibuprofen degradation by visible light assisted photocatalytic activation of persulfate. Surf. Interfaces 2022, 35, 102472. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.-Q.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, Y.; Lin, H.; Jie, B.; Zheng, Z.; Zhang, X. Bicarbonate-enhanced iron-based Prussian blue analogs catalyze the Fenton-like degradation of p-nitrophenol. J. Colloid Interface Sci. 2022, 608, 2884–2895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Lin, N.; Xu, J.; Liu, X.; Liu, N.; Zhang, X. Degradation of norfloxacin by MOF-derived lamellar carbon nanocomposites based on microwave-driven Fenton reaction: Improved Fe(III)/Fe(II) cycle. Chemosphere 2022, 293, 133614. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Gong, Y.; Wang, R.; Wang, Y.; Zhang, X. Critical review of perovskite-based materials in advanced oxidation system for wastewater treatment: Design, applications and mechanisms. J. Hazard. Mater. 2022, 424, 127637. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, K.; Rao, R.; Chen, J.; Liu, Q.; Yang, Y.; Bi, F.; Wang, Y.; Xu, J.; Liu, N. Synthesis of acidic MIL-125 from plastic waste: Significant contribution of N orbital for efficient photocatalytic degradation of chlorobenzene and toluene. Appl. Catal. B 2022, 310, 121300. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, W.; Li, X.; Lin, H.; Chen, H.; Bi, F.; Zheng, Z.; Xu, J.; Zhang, X. Insights into the mechanism of enhanced peroxymonosulfate degraded tetracycline using metal organic framework derived carbonyl modified carbon-coated Fe0. J. Hazard. Mater. 2022, 424, 127640. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Bi, F.; Chen, J.; Li, Y.; Cui, L.; Xu, J.; Zhang, X. Oxygen-vacancy-induced O2 activation and electron-hole migration enhance photothermal catalytic toluene oxidation. Cell Rep. Phys. Sci. 2022, 3, 101011. [Google Scholar] [CrossRef]
- Pan, Q.; Gao, Q.; Gao, G.; Liu, M.; Han, B.; Xia, K.; Zhou, C. Composition-engineered LaCoO3-based monolithic catalysts for easily operational and robust peroxymonosulfate activation. Chem. Eng. J. 2021, 424, 130574. [Google Scholar] [CrossRef]
- Gao, P.; Yan, S.; Tian, X.; Nie, Y.; Wang, Y.; Deng, Y.; Tu, J. Identification and manipulation of active centers on perovskites to enhance catalysis of peroxymonosulfate for degradation of emerging pollutants in water. J. Hazard. Mater. 2022, 424, 127384. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Aluru, N. The role of A-site ion on proton diffusion in perovskite oxides (ABO3). J. Power Sources 2020, 445, 227327. [Google Scholar] [CrossRef]
- Rischau, C.W.; Lin, X.; Grams, C.P.; Finck, D.; Harms, S.; Engelmayer, J.; Lorenz, T.; Gallais, Y.; Fauqué, B.; Hemberger, J.; et al. A ferroelectric quantum phase transition inside the superconducting dome of Sr1−xCaxTiO3−δ. Nat. Phys. 2017, 13, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Di, J.; Xia, J.; Zhu, W.; Li, H. Surface Defect Engineering in 2D Nanomaterials for Photocatalysis. Adv. Funct. Mater. 2018, 28, 1801983. [Google Scholar] [CrossRef]
- Geng, G.; Cai, M.; Fang, R.; Luan, Q.; Zhang, Z.; Song, J.; Zhang, J. Metal-organic frameworks-derived perovskite catalysts for efficient degradation of 2, 4-dichlorophenol via peroxymonosulfate activation. Appl. Surf. Sci. 2020, 534, 147467. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y.; Zhu, K.; Zhang, H. Understanding oxygen-deficient La2CuO4-δperovskite activated peroxymonosulfate for bisphenol A degradation: The role of localized electron within oxygen vacancy. Appl. Catal. B 2021, 284, 119732. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Du, B.; Wang, Y.; Xiong, Y.; Yang, Y.; Zhang, X. Synthesis of hierarchically porous perovskite-carbon aerogel composite catalysts for the rapid degradation of fuchsin basic under microwave irradiation and an insight into probable catalytic mechanism. Appl. Surf. Sci. 2018, 439, 475–487. [Google Scholar] [CrossRef]
- Palas, B.; Ersöz, G.; Atalay, S. Catalytic wet air oxidation of Reactive Black 5 in the presence of LaNiO3 perovskite catalyst as a green process for azo dye removal. Chemosphere 2018, 209, 823–830. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Gong, Y.; Lin, N.; Yang, Q.; Zhang, X.; Wang, Y. Perovskite catalysts with different dimensionalities for environmental and energy applications: A review. Sep. Purif. Technol. 2023, 307, 122716. [Google Scholar] [CrossRef]
- Lin, H.; Jie, B.; Ye, J.; Zhai, Y.; Luo, Z.; Shao, G.; Chen, R.; Zhang, X.; Yang, Y. Recent advance of macroscopic metal-organic frameworks for water treatment: A review. Surfaces Interfaces 2023, 36, 102564. [Google Scholar] [CrossRef]
- Tahini, H.A.; Tan, X.; Schwingenschlögl, U.; Smith, S.C. Formation and Migration of Oxygen Vacancies in SrCoO3 and Their Effect on Oxygen Evolution Reactions. ACS Catal. 2016, 6, 5565–5570. [Google Scholar] [CrossRef] [Green Version]
- Run, Z.; Hao, Y. Oxygen vacancies induced tuning effect on physical properties of multiferroic perovskite oxide thin films. Acta Phys. Sin. 2018, 67, 156101. [Google Scholar] [CrossRef]
- Wang, K.; Han, C.; Shao, Z.; Qiu, J.; Wang, S.; Liu, S. Perovskite Oxide Catalysts for Advanced Oxidation Reactions. Adv. Funct. Mater. 2021, 31, 2102089. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Baiyee, Z.M.; Shao, Z.; Ciucci, F. Nonstoichiometric Oxides as Low-Cost and Highly-Efficient Oxygen Reduction/Evolution Catalysts for Low-Temperature Electrochemical Devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef] [PubMed]
- Yaremchenko, A.A.; Populoh, S.; Patrício, S.G.; Macías, J.; Thiel, P.; Fagg, D.P.; Weidenkaff, A.; Frade, J.R.; Kovalevsky, A.V. Boosting Thermoelectric Performance by Controlled Defect Chemistry Engineering in Ta-Substituted Strontium Titanate. Chem. Mater. 2015, 27, 4995–5006. [Google Scholar] [CrossRef]
- Aidhy, D.S.; Liu, B.; Zhang, Y.; Weber, W.J. Chemical expansion affected oxygen vacancy stability in different oxide structures from first principles calculations. Comput. Mater. Sci. 2015, 99, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.J.; Liu, Y.S.; Yang, C.P.; Chen, S.S.; Tang, S.L.; Bärner, K. Oxygen vacancy related defect dipoles in CaCu3Ti4O12: Detected by electron paramagnetic resonance spectroscopy. J. Eur. Ceram. Soc. 2015, 35, 2073–2081. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, L.; Lan, C.; Pandey, S.S.; Hayase, S.; Ma, T. Oxygen vacancy formation and migration in double perovskite Sr2CrMoO6: A first-principles study. RSC Adv. 2016, 6, 43034–43040. [Google Scholar] [CrossRef]
- Jia, Y.; Miglio, A.; Gonze, X.; Mikami, M. Ab-initio study of oxygen vacancy stability in bulk and Cerium-doped lutetium oxyorthosilicate. J. Lumin 2018, 204, 499–505. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Non-Stoichiometric Redox Active Perovskite Materials for Solar Thermochemical Fuel Production: A Review. Catalysts 2018, 8, 611. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Kuang, H.; Zhang, H.R.; Zhao, Y.Y.; Qiao, K.M.; Wang, F.; Liu, W.; Wang, W.; Peng, L.C.; et al. Oxygen defect engineering by the current effect assisted with temperature cycling in a perovskite-type La0.7Sr0.3CoO3 film. Nanoscale 2017, 9, 13214–13221. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Škapin, S.; Ubic, R. Correlative models for oxygen vacancies in perovskites. J. Alloy. Compd. 2020, 836, 155475. [Google Scholar] [CrossRef]
- Li, B.; Yang, Q.; Peng, Y.; Chen, J.; Deng, L.; Wang, D.; Hong, X.; Li, J. Enhanced low-temperature activity of LaMnO3 for toluene oxidation: The effect of treatment with an acidic KMnO4. Chem. Eng. J. 2019, 366, 92–99. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Zhao, S.; Xiang, S.; Gao, W.; Wang, L.; Xu, J.; Wang, Y. The promoting effect of alkali metal and H2O on Mn-MOF derivatives for toluene oxidation: A combined experimental and theoretical investigation. J. Catal. 2022, 415, 218–235. [Google Scholar] [CrossRef]
- Du, X.; Li, C.; Zhao, L.; Zhang, J.; Gao, L.; Sheng, J.; Yi, Y.; Chen, J.; Zeng, G. Promotional removal of HCHO from simulated flue gas over Mn-Fe oxides modified activated coke. Appl. Catal. B 2018, 232, 37–48. [Google Scholar] [CrossRef]
- Gunkel, F.; Christensen, D.V.; Chen, Y.Z.; Pryds, N. Oxygen vacancies: The (in)visible friend of oxide electronics. Appl. Phys. Lett. 2020, 116, 120505. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.V.; Trier, F.; Niu, W.; Gan, Y.; Zhang, Y.; Jespersen, T.S.; Chen, Y.; Pryds, N. Stimulating Oxide Heterostructures: A Review on Controlling SrTiO 3 -Based Heterointerfaces with External Stimuli. Adv. Mater. Interfaces 2019, 6, 1900772. [Google Scholar] [CrossRef] [Green Version]
- Pryds, N.; Esposito, V. When two become one: An insight into 2D conductive oxide interfaces. J. Electroceramics 2016, 38, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yang, Y.; Bi, F.; Chen, Y.; Wu, M.; Zhang, X.; Wang, G. Oxygen vacancies in the catalyst: Efficient degradation of gaseous pollutants. Chem. Eng. J. 2023, 454, 140376. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, B.; Xiong, T.; Jiang, D.; Ren, X.; Lu, P.; Fu, M. Enhanced visible light driven photocatalytic performance of Bi2WO6 nano-catalysts by introducing oxygen vacancy. J. Alloy. Compd. 2021, 887, 161297. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Shi, W.; Ling, P.; Sun, Y.; Jiao, X.; Gao, S.; Liang, L.; Xu, J.; Yan, W.; et al. Efficient Visible-Light-Driven CO 2 Reduction Mediated by Defect-Engineered BiOBr Atomic Layers. Angew. Chem. Int. Ed. 2018, 57, 8719–8723. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, M.; Jin, X.; Tian, J.; Li, M.; Zhou, Y.; Zhang, L.; Li, Y.; Shi, J. Oxygen Vacancy Generation and Stabilization in CeO2–x by Cu Introduction with Improved CO2 Photocatalytic Reduction Activity. ACS Catal. 2019, 9, 4573–4581. [Google Scholar] [CrossRef]
- Chu, K.; Liu, F.; Zhu, J.; Fu, H.; Zhu, H.; Zhu, Y.; Zhang, Y.; Lai, F.; Liu, T. A General Strategy to Boost Electrocatalytic Nitrogen Reduction on Perovskite Oxides via the Oxygen Vacancies Derived from A-Site Deficiency. Adv. Energy Mater. 2021, 11, 2003799. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Yu, L.; Wang, Y.; Zhang, C.; Zhang, X. Efficient reactivity of LaCu0.5Co0.5O3 perovskite intercalated montmorillonite and g-C3N4 nanocomposites in microwave-induced H2O2 catalytic degradation of bisphenol A. Chem. Eng. J. 2020, 401, 126057. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, J.; Zhang, Y.; Wang, Z. Tuning oxygen vacancies on LaFeO3 perovskite as efficient electrocatalysts for oxygen evolution reaction. Mater. Lett. 2021, 309, 131317. [Google Scholar] [CrossRef]
- Gu, W.; Wang, W.; Li, G.; Xie, H.; Wong, P.K.; An, T. Microwave-assisted synthesis of defective tungsten trioxide for photocatalytic bacterial inactivation: Role of the oxygen vacancy. Chin. J. Catal. 2020, 41, 1488–1497. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Zang, Y.; Liu, C.; Wang, W.; Han, R.; Ji, N.; Zhang, S.; Liu, Q. Promotion of A-Site Ag-Doped Perovskites for the Catalytic Oxidation of Soot: Synergistic Catalytic Effect of Dual Active Sites. ACS Catal. 2021, 11, 14224–14236. [Google Scholar] [CrossRef]
- Zhang, P.; Mei, X.; Zhao, X.; Xiong, J.; Li, Y.; Zhao, Z.; Wei, Y. Boosting Catalytic Purification of Soot Particles over Double Perovskite-Type La2–xKxNiCoO6 Catalysts with an Ordered Macroporous Structure. Environ. Sci. Technol. 2021, 55, 11245–11254. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, Y.; Hu, Y.; Huang, B.; Sun, M.; Da, P.; Xi, P.; Yan, C.-H. Controlling the Cation Exsolution of Perovskite to Customize Heterostructure Active Site for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2022, 14, 25638–25647. [Google Scholar] [CrossRef]
- Pang, S.; Yang, G.; Jiang, X.; Shen, X.; Rao, D.; Chen, C. Insight into tuning the surface and bulk microstructure of perovskite catalyst through control of cation non-stoichiometry. J. Catal. 2020, 381, 408–414. [Google Scholar] [CrossRef]
- Kim, K.; Joo, S.; Huang, R.; Kim, H.J.; Kim, G.; Han, J.W. Mechanistic insights into the phase transition and metal ex-solution phenomena of Pr0.5Ba0.5Mn0.85Co0.15O3−δ from simple to layered perovskite under reducing conditions and enhanced catalytic activity. Energy Environ. Sci. 2021, 14, 873–882. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Shi, L.; Hoang, S.; Yang, W.; Fang, Y.; Liang, Z.; Pan, C.; Zhu, Y.; Li, L.; et al. Oxygen Vacancies and Lewis Acid Sites Synergistically Promoted Catalytic Methane Combustion over Perovskite Oxides. Environ. Sci. Technol. 2021, 55, 9243–9254. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ma, X.; Miao, J.; Ran, R.; Zhou, W.; Wang, S.; Shao, Z. Nonstoichiometric perovskite for enhanced catalytic oxidation through excess A-site cation. Chem. Eng. Sci. 2020, 219, 115596. [Google Scholar] [CrossRef]
- Liang, P.; Meng, D.; Liang, Y.; Wang, Z.; Zhang, C.; Wang, S.; Zhang, Z. Cation deficiency tuned LaCoO3−δ perovskite for peroxymonosulfate activation towards bisphenol A degradation. Chem. Eng. J. 2020, 409, 128196. [Google Scholar] [CrossRef]
- Islam, Q.A.; Majee, R.; Bhattacharyya, S. Bimetallic nanoparticle decorated perovskite oxide for state-of-the-art trifunctional electrocatalysis. J. Mater. Chem. A 2019, 7, 19453–19464. [Google Scholar] [CrossRef]
- Ren, H.; Wang, Z.; Chen, X.; Jing, Z.; Qu, Z.; Huang, L. Effective mineralization of p-nitrophenol by catalytic ozonation using Ce-substituted La1−xCexFeO3 catalyst. Chemosphere 2021, 285, 131473. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, Y.; Lin, N.; Yu, L.; Du, B.; Zhang, X. Enhanced removal of Cr(VI) from aqueous solution by stabilized nanoscale zero valent iron and copper bimetal intercalated montmorillonite. J. Colloid Interface Sci. 2022, 606, 941–952. [Google Scholar] [CrossRef]
- Wu, M.; Li, H.; Ma, S.; Chen, S.; Xiang, W. Boosting the surface oxygen activity for high performance Iron-based perovskite oxide. Sci. Total. Environ. 2021, 795, 148904. [Google Scholar] [CrossRef]
- Ding, J.; Liu, J.; Yang, Y.; Zhao, L.; Yu, Y. Understanding A-site tuning effect on formaldehyde catalytic oxidation over La-Mn perovskite catalysts. J. Hazard. Mater. 2022, 422, 126931. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, Y.; Gao, Z.; Tian, Y.; Zhang, J.; Jiang, Z.; Li, X. A-site defects in LaSrMnO3 perovskite-based catalyst promoting NO storage and reduction for lean-burn exhausts. J. Rare Earths 2021, 39, 959–968. [Google Scholar] [CrossRef]
- Han, P.; Li, X.; Jin, C.; Tan, X.; Sun, W.; Wang, S.; Ding, F.; Li, X.; Jin, H.; Sun, C.; et al. Cation deviated stoichiometry Ca1.1ZrO3 perovskite as an efficient ozonation catalyst for m-cresol wastewater degradation. Chem. Eng. J. 2022, 429, 132218. [Google Scholar] [CrossRef]
- You, M.; Gui, L.; Ma, X.; Wang, Z.; Xu, Y.; Zhang, J.; Sun, J.; He, B.; Zhao, L. Electronic tuning of SrIrO3 perovskite nanosheets by sulfur incorporation to induce highly efficient and long-lasting oxygen evolution in acidic media. Appl. Catal. B 2021, 298, 120562. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-metal fluorine doping in Ruddlesden–Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Saloaro, M.; Liedke, M.; Angervo, I.; Butterling, M.; Hirschmann, E.; Wagner, A.; Huhtinen, H.; Paturi, P. Exploring the anti-site disorder and oxygen vacancies in Sr2FeMoO6 thin films. J. Magn. Magn. Mater. 2021, 540, 168454. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Yu, Y.; Cai, X.; Gao, T.; Zhang, T.; Sun, H.; Gu, C.; Gu, Z.; Zhu, Y.; et al. Rewritable High-Mobility Electrons in Oxide Heterostructure of Layered Perovskite/Perovskite. ACS Appl. Mater. Interfaces 2021, 13, 7812–7821. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Bian, J.; Wang, L.; Wang, J.; Du, Y.; Wang, Z.; Wu, C.; Yang, Y. Enhanced photocatalytic activity of perovskite NaNbO3 by oxygen vacancy engineering. Phys. Chem. Chem. Phys. 2019, 21, 11697–11704. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, J.; Fu, J.; Jiang, G.; Lui, G.; Luo, D.; Deng, Y.; Zhang, J.; Cano, Z.P.; Yu, A.; et al. An Oxygen-Vacancy-Rich Semiconductor-Supported Bifunctional Catalyst for Efficient and Stable Zinc–Air Batteries. Adv. Mater. 2018, 31, 1806761. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Study on Defect Regulation and Photocatalytic and Electrocatalytic Properties in Transition Metal Compounds; University of Science and Technology of China: Hefei, China, 2021; p. 000502. [Google Scholar]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.-B.; Yang, Z.; Zheng, G. Reduced Mesoporous Co3O4Nanowires as Efficient Water Oxidation Electrocatalysts and Supercapacitor Electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Sun, W.; Wei, H.; An, L.Y.; Jin, C.; Wu, H.; Xiong, Z.-A.; Pu, C.; Sun, C. Oxygen vacancy mediated La1-xCexFeO3-δ perovskite oxides as efficient catalysts for CWAO of acrylic acid by A-site Ce doping. Appl. Catal. B 2019, 245, 20–28. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Chen, P.; Zhou, G.; Liu, Z.; Xu, Y. Preparation of Supported Perovskite Catalyst to Purify Membrane Concentrate of Coal Chemical Wastewater in UV-Catalytic Wet Hydrogen Peroxide Oxidation System. Int. J. Environ. Res. Public Heal. 2021, 18, 4906. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.-S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T.S. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, X.; Chu, X.; Cao, R.; Qian, J.; Cong, Y.; Huang, K.; Wang, J.; Redshaw, C.; Sarangi, R.; et al. Manipulating Surface Termination of Perovskite Manganate for Oxygen Activation. Adv. Funct. Mater. 2021, 31, 2006439. [Google Scholar] [CrossRef]
- Kim, K.; Koo, B.; Jo, Y.-R.; Lee, S.; Kim, J.K.; Kim, B.-J.; Jung, W.; Han, J.W. Control of transition metal–oxygen bond strength boosts the redox ex-solution in a perovskite oxide surface. Energy Environ. Sci. 2020, 13, 3404–3411. [Google Scholar] [CrossRef]
- Oh, J.H.; Kwon, B.W.; Cho, J.; Lee, C.H.; Kim, M.K.; Choi, S.H.; Yoon, S.P.; Han, J.H.; Nam, S.W.; Kim, J.Y.; et al. Importance of Exsolution in Transition-Metal (Co, Rh, and Ir)-Doped LaCrO3Perovskite Catalysts for Boosting Dry Reforming of CH4Using CO2for Hydrogen Production. Ind. Eng. Chem. Res. 2019, 58, 6385–6393. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Yang, J.; Yu, Q.; Zhu, X.; Chu, J.; Du, Y.; Wang, C.; Hua, Y.; Li, H.; et al. Plasma treated Bi2WO6 ultrathin nanosheets with oxygen vacancies for improved photocatalytic CO2 reduction. Inorg. Chem. Front. 2020, 7, 597–602. [Google Scholar] [CrossRef]
- Tong, B.; Meng, G.; Deng, Z.; Horprathum, M.; Klamchuen, A.; Fang, X. Surface oxygen vacancy defect engineering of p-CuAlO2via Ar&H2 plasma treatment for enhancing VOCs sensing performances. Chem. Commun. 2019, 55, 11691–11694. [Google Scholar] [CrossRef]
- Selvadurai, A.P.B.; Xiong, T.; Huang, P.; Tan, Q.; Huang, Y.; Yang, H.; Balogun, M.-S.J.T. Tailoring the cationic and anionic sites of LaFeO3-based perovskite generates multiple vacancies for efficient water oxidation. J. Mater. Chem. A 2021, 9, 16906–16916. [Google Scholar] [CrossRef]
- Mishra, A.; Li, T.; Li, F.; Santiso, E.E. Oxygen Vacancy Creation Energy in Mn-Containing Perovskites: An Effective Indicator for Chemical Looping with Oxygen Uncoupling. Chem. Mater. 2018, 31, 689–698. [Google Scholar] [CrossRef]
- Kang-Wen, Q.; Xi, C.; Zhang, Y.; Zhang, R.; Li, Z.; Sheng, G.-R.; Liu, H.; Dong, C.-K.; Chen, Y.-J.; Du, X.-W. Laser-induced oxygen vacancies in FeCo2O4 nanoparticles for boosting oxygen evolution and reduction. Chem. Commun. 2019, 55, 8579–8582. [Google Scholar] [CrossRef]
- Haselmann, U.; Suyolcu, Y.E.; Wu, P.-C.; Ivanov, Y.P.; Knez, D.; van Aken, P.A.; Chu, Y.-H.; Zhang, Z. Negatively Charged In-Plane and Out-Of-Plane Domain Walls with Oxygen-Vacancy Agglomerations in a Ca-Doped Bismuth-Ferrite Thin Film. ACS Appl. Electron. Mater. 2021, 3, 4498–4508. [Google Scholar] [CrossRef] [PubMed]
- Badreldin, A.; Abusrafa, A.E.; Abdel-Wahab, A. Oxygen-deficient perovskites for oxygen evolution reaction in alkaline media: A review. Emergent Mater. 2020, 3, 567–590. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Ren, F.; Bai, Y.; Cheng, Z. Smart oxygen vacancy engineering to enhance water oxidation efficiency by separating the different effects of bulk and surface vacancies. Mater. Today Energy 2021, 19, 100619. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, M.; Yang, X.; Yu, X.; Ge, M. Boosting benzene combustion by engineering oxygen vacancy-mediated Ag/CeO2-Co3O4 catalyst via interfacial electron transfer. J. Colloid Interface Sci. 2021, 594, 882–890. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Liu, Y.; Zhu, X.; Yang, W. Selection of oxygen permeation models for different mixed ionic-electronic conducting membranes. AIChE J. 2017, 63, 4043–4053. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; Lei, L.; Huang, K. Ta-Doped SrCoO3−δ as a promising bifunctional oxygen electrode for reversible solid oxide fuel cells: A focused study on stability. J. Mater. Chem. A 2017, 5, 8989–9002. [Google Scholar] [CrossRef]
- Asnavandi, M.; Yin, Y.; Li, Y.; Sun, C.; Zhao, C. Promoting Oxygen Evolution Reactions through Introduction of Oxygen Vacancies to Benchmark NiFe–OOH Catalysts. ACS Energy Lett. 2018, 3, 1515–1520. [Google Scholar] [CrossRef]
- Wu, M.; Luo, M.; Guo, M.; Jia, L. Sugarcane Bagasse Hydrolysis by Metal Ions Mediated Synthesis of Perovskite LaCoO3 and the Photocatalytic Performance for Hydrogen from Formaldehyde Solution under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 11558–11565. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, H.; Kannan, A.M.; Pérez-Salcedo, K.; Escobar, B. Effect of Thermally Induced Oxygen Vacancy of α-MnO2 Nanorods toward Oxygen Reduction Reaction. Inorg. Chem. 2019, 58, 5335–5344. [Google Scholar] [CrossRef]
- Ma, M.; Peng, L.; Li, J.; Zhang, Y.; Wang, Z.; Bi, J.; Gao, D.; Wu, J. Oxygen vacancy engineering and superior sensing properties of hematite prepared via a one-step treatment. Sensors Actuators B 2021, 339, 129907. [Google Scholar] [CrossRef]
- Liang, J.; Xu, Q.; Teng, X.; Guan, W.; Lu, C. Superoxide-Triggered Luminol Electrochemiluminescence for Detection of Oxygen Vacancy in Oxides. Anal. Chem. 2020, 92, 1628–1634. [Google Scholar] [CrossRef]
- Magray, M.A.; Ikram, M.; Najim, M. Impact of oxygen vacancies to control the magnetic and electronic properties of the La2CoMnO6 system. J. Magn. Magn. Mater. 2021, 529, 167857. [Google Scholar] [CrossRef]
- Tran, S.B.T.; Choi, H.; Oh, S.; Park, J.Y. Defective Nb2O5-supported Pt catalysts for CO oxidation: Promoting catalytic activity via oxygen vacancy engineering. J. Catal. 2019, 375, 124–134. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Liu, B. Effect of oxygen vacancies on ceria catalyst for selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2020, 529, 147068. [Google Scholar] [CrossRef]
- Zhai, Z.; Yan, W.; Dong, L.; Deng, S.; Wilkinson, D.P.; Wang, X.; Zhang, L.; Zhang, J. Catalytically active sites of MOF-derived electrocatalysts: Synthesis, characterization, theoretical calculations, and functional mechanisms. J. Mater. Chem. A 2021, 9, 20320–20344. [Google Scholar] [CrossRef]
- Wang, H.B.; Liang, W.; Luo, Z.L.; Liu, Q.Q.; Huang, H.L.; Yang, M.M.; Yang, Y.J.; Hu, S.X.; Jiang, Z.; Li, S.; et al. Location of oxygen vacancies in Sr2CuO3+δ single crystal determined by polarized EXAFS at Cu K-edge. Solid State Commun. 2019, 299, 113649. [Google Scholar] [CrossRef]
- Xie, J.; Bai, P.; Wang, C.; Chen, N.; Chen, W.; Duan, M.; Wang, H. Enhancing H2 Evolution by Adjusting Oxygen Species at Perovskite SrTiO3. ACS Appl. Energy Mater. 2022, 5, 9559–9570. [Google Scholar] [CrossRef]
- Jing, J.; Pervez, N.; Sun, P.; Cao, C.; Li, B.; Naddeo, V.; Jin, W.; Zhao, Y. Highly efficient removal of bisphenol A by a novel Co-doped LaFeO3 perovskite/PMS system in salinity water. Sci. Total. Environ. 2021, 801, 149490. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; Yao, G.; Lai, B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Fang, X.; Cheng, Y. Simple strategy for the construction of oxygen vacancies on alpha-MnO2 catalyst to improve toluene catalytic oxidation. J. Hazard. Mater. 2021, 409, 125020. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, H.; Liu, Z.; Liu, X.; Zhou, G.; Jiang, Y. Oxygen vacancy induced MnO2 catalysts for efficient toluene catalytic oxidation. Catal. Sci. Technol. 2021, 11, 6708–6723. [Google Scholar] [CrossRef]
- Yang, Q.; Du, J.; Li, J.; Wu, Y.; Zhou, Y.; Yang, Y.; Yang, D.; He, H. Thermodynamic and Kinetic Influence of Oxygen Vacancies on the Solar Water Oxidation Reaction of alpha-Fe2O3 Photoanodes. ACS Appl. Mater. Interfaces 2020, 12, 11625–11634. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Niu, F.; Qin, L.; Wang, S.; Zhang, N.; Huang, Y. Defective BiFeO3 with surface oxygen vacancies: Facile synthesis and mechanism insight into photocatalytic performance. Sol. Energy Mater. Sol. Cells 2017, 171, 24–32. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, Y.I.; Kim, K.; Kim, J.C.; Lee, J.H.; Park, S.S.; Han, J.W.; Kim, Y.M.; Kim, G.; Jeong, H.Y. Probing One-Dimensional Oxygen Vacancy Channels Driven by Cation–Anion Double Ordering in Perovskites. Nano Lett. 2020, 20, 8353–8359. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, X.; Jing, M.; Song, W.; Liu, J.; Ge, M. Defective MnxZr1–xO2 Solid Solution for the Catalytic Oxidation of Toluene: Insights into the Oxygen Vacancy Contribution. ACS Appl. Mater. Interfaces 2019, 11, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, G.; Zhang, Y.; Liu, Y. Oxygen vacancies boosted Co-Co2C catalysts for higher alcohols synthesis from syngas. Appl. Surf. Sci. 2022, 576, 151846. [Google Scholar] [CrossRef]
- Rao, Y.; Zhang, Y.; Han, F.; Guo, H.; Huang, Y.; Li, R.; Qi, F.; Ma, J. Heterogeneous activation of peroxymonosulfate by LaFeO3 for diclofenac degradation: DFT-assisted mechanistic study and degradation pathways. Chem. Eng. J. 2018, 352, 601–611. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Jie, B.; Zheng, Z.; Li, J.; Zhu, C.; Wang, S.; Xu, J.; Zhang, X. Electron structure modulation and bicarbonate surrounding enhance Fenton-like reactions performance of Co-Co PBA. J. Hazard. Mater. 2022, 437, 129372. [Google Scholar] [CrossRef]
- Liu, N.; Dai, W.; Fei, F.; Xu, H.; Lei, J.; Quan, G.; Zheng, Y.; Zhang, X.; Tang, L. Insights into the photocatalytic activation persulfate by visible light over ReS2/MIL-88B(Fe) for highly efficient degradation of ibuprofen: Combination of experimental and theoretical study. Sep. Purif. Technol. 2022, 297, 121545. [Google Scholar] [CrossRef]
- Liu, N.; Fei, F.; Dai, W.; Lei, J.; Bi, F.; Wang, B.; Quan, G.; Zhang, X.; Tang, L. Visible-light-assisted persulfate activation by SnS2/MIL-88B(Fe) Z-scheme heterojunction for enhanced degradation of ibuprofen. J. Colloid Interface Sci. 2022, 625, 965–977. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Nie, Z.; Ma, L.; Xi, X.; Liu, Y.; Zhao, L. First-principles Study on Nanoscale Tungsten Oxide: A Review. J. Inorg. Mater. 2021, 36, 1125. [Google Scholar] [CrossRef]
- Hoffmann, M.; Antonov, V.N.; Bekenov, L.V.; Kokko, K.; Hergert, W.J.; Ernst, A. Variation of magnetic properties of Sr2FeMoO6 due to oxygen vacancies. J. Phys. Condens. Matter 2018, 30, 305801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Cheng, J.; Zhang, Q.; Tang, W.; Yang, K. Creating a two-dimensional hole gas in a polar/polar LaAlO3/KTaO3 perovskite heterostructure. J. Mater. Chem. C 2020, 8, 14230–14237. [Google Scholar] [CrossRef]







| Perovskite | Catalyst Dosage | AOPs | Organic Pollutants | Catalyst Rate | Ref. |
|---|---|---|---|---|---|
| Bi2WO6 | 50 mL of 20 mg/L CIP aqueous solution. | Photocatalyst | ciprofloxacin | 90% at 6 h | [13] |
| LaCu0.5Co0.5O3-MMT0.2/CNx | 9.5 g/L | Microwave irradiation | Bisphenol A | 98.7% at 6 min | [14] |
| LaCoO3 | 10 mg of 50 mg/L 2,4-dichlorophenol | Sulfate Radical-Based AOPs | 2,4-Dichlorophenol | 99.8% at 25 min | [15] |
| BiFeO3 | 0.5 g/L | Fenton-like | Rhodamine B | 95.2% at 90 min | [12] |
| LaMn4Ox | 0.2 g/L | Ozonation | Oxalic acid | 100% at 45 min | [12] |
| La2CuO4−δ | 0.7 g/L | Sulfate Radical-Based AOPs | Bisphenol A | 96.7% at 60 min | [16] |
| LaFe0.5M0.5O3-CA (M = Mn, Cu) | 0.4 g/L | Microwave irradiation | Fuchsin basic | 100% at 4 min | [17] |
| LaNiO3 | 1 g/L | Wet air oxidation | Reactive Black | 65.4% at 120 min | [18] |
| Classification | Perovskite | Preparation Method | Organic Pollutants | Synthesis Parameters | Catalyst Rate | Ref. |
|---|---|---|---|---|---|---|
| Cation substitutions: A-cation | La1−xAgxCoO3 | EDTA−citric acid complexation | Soot | LaCoO3 was partially replaced with Ag+. PH = 7, Calcination: 700 °C, 5 h | 99% | [45] |
| La2−xKxNiCoO6 | Colloidal crystal template | Soot | The partial substitution of A-site (La) with low-valence potassium (K) ions. Calcination: 346 °C | 99.3% | [46] | |
| La2NiFeO6 | Modified sol–gel method (Pechini method) | Toluene | Heating:180 °C, 10 h Drying: 70 °C, 1 h Calcination: 650 °C, 5 h; | 90% | [47] | |
| Cation substitutions: B-cation | LaCoO3−δ | Sol–gel method | Bisphenol A | Drying: 60 °C, overnight annealed in air: 700 °C, 2 h Calcination: 800 °C | 90% | [48] |
| Ion non-stoichiometry | Ca1.1ZrO3 | coprecipitation calcination method | M-cresol | Heating:100 °C, Drying: 120 °C, 12 h Calcination: 900/1100 °C, 6 h; | 100% | [49] |
| La1.15MnO3+d | Sol–gel method | Rhodamine B | pre-decomposed at 180 °C, for 6 h Calcination: 800 °C, 5 h; | 100% | [50] | |
| Chemical reduction | NaNbO3 | Solid–state reaction | Methyl blue (MB) | Calcination: 325–500 °C, 61 h, N2 atmosphere Heating rate:5 °C min−1 Drying: 60 °C, vacuum atmosphere | Enhanced by almost 2.4 times | [50] |
| Exsolution | Pr0.5Ba0.5Mn0.85Co0.15 | B-metal ex-solution | CO | Heating: 300 °C, Calcination: 600 °C, 4 h | 82.5% | [51] |
| Etching | La0.8Sr0.2CoO3 | Etching by Oxalic acid | CH4 | Calcination: 700 °C, 2 h; Drying: 55 °C | - | [52] |
| Mathod | Ways to Detecting Oxygen Vacancies | Qualitative or Quantitative | Reference |
|---|---|---|---|
| X-ray diffraction (XRD) | Detect crystal structure and lattice parameters | Qualitative | [78,50,79] |
| X-ray photoelectron spectroscopy (XPS) | Elemental composition and chemical environment near the surface | Qualitative | [55,72,80] |
| Transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM) | Crystal structure and atomic arrangements | Qualitative | [81,82] |
| Raman spectra | A peak shift and/or the presence of additional peak | Qualitative | [49,78] |
| Ultraviolet-visible spectroscopy (UV-vis) | Peak changes | Qualitative | [76] |
| Thermogravimetric analysis (TGA) | Weight changes | Quantitative | [78,79] |
| X-ray Absorbtion Spectra (XAS), X-ray absorption fine structure (EXAFS) and X-ray absorption near-edge structure (XANES) | Variations of XAS peaks | Qualitative | [49,83] |
| Electron Paramagnetic Resonance (EPR) | Valuable information about unpaired electrons | Qualitative | [16,45,82,61] |
| H2-TPR, O2-TPD | Chemical absorption oxygen/the redox potential | Qualitative | [16,84] |
| Iodometric titration (IT) | Quantitative analysis oxygen Stoichiometry | Quantitative | [85,86] |
| Photoluminescence (PL) Spectroscopy | Electronic structure | Qualitative | [87,88] |
| Electron energy-loss spectroscopy (EELS) | Content and electronic structures | Qualitative | [25,89] |
| Neutron powder diffraction (NPD) | Crystal structures | Qualitative | [82,90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Li, H.; Lin, N.; Gong, Y.; Jiang, H.; Chen, J.; Wang, Y.; Zhang, X. Oxygen-Deficient Engineering for Perovskite Oxides in the Application of AOPs: Regulation, Detection, and Reduction Mechanism. Catalysts 2023, 13, 148. https://doi.org/10.3390/catal13010148
Yu J, Li H, Lin N, Gong Y, Jiang H, Chen J, Wang Y, Zhang X. Oxygen-Deficient Engineering for Perovskite Oxides in the Application of AOPs: Regulation, Detection, and Reduction Mechanism. Catalysts. 2023; 13(1):148. https://doi.org/10.3390/catal13010148
Chicago/Turabian StyleYu, Jiayu, Huanhuan Li, Naipeng Lin, Yishu Gong, Hu Jiang, Jiajia Chen, Yin Wang, and Xiaodong Zhang. 2023. "Oxygen-Deficient Engineering for Perovskite Oxides in the Application of AOPs: Regulation, Detection, and Reduction Mechanism" Catalysts 13, no. 1: 148. https://doi.org/10.3390/catal13010148
APA StyleYu, J., Li, H., Lin, N., Gong, Y., Jiang, H., Chen, J., Wang, Y., & Zhang, X. (2023). Oxygen-Deficient Engineering for Perovskite Oxides in the Application of AOPs: Regulation, Detection, and Reduction Mechanism. Catalysts, 13(1), 148. https://doi.org/10.3390/catal13010148