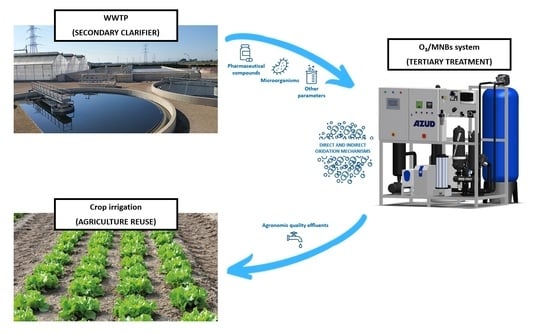
Full-Scale O3/Micro-Nano Bubbles System Based Advanced Oxidation as Alternative Tertiary Treatment in WWTP Effluents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pharmaceuticals Removal Efficiency
2.2. Prediction of the Pharmaceutical Removal from Physico-Chemical Properties
2.3. Transformation Products Generated in the Process
2.4. Agronomic Quality of System Effluents under Optimal Operation Conditions
2.5. Cost Assessment Approach
3. Materials and Methods
3.1. System Design
3.2. Pharmaceutical Compounds Included in the Study
3.3. Analytical Determinations
3.4. Experimental Procedure and Sampling
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the aquatic environment: A review on eco-toxicology and the remediation potential of algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef]
- Aitken, M.; Kleinrock, M.; Munoz, E. Global Medicine Spending and Usage Trends: Outlook to 2025; IQVIA Institute for Human Data Science: Parsippany, NJ, USA, 2021. [Google Scholar]
- Ayati, N.; Saiyarsarai, P.; Nikfar, S. Short and long-term impacts of COVID-19 on the pharmaceutical sector. DARU J. Pharm. Sci. 2020, 28, 799–805. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Lindim, C.; Van Gils, J.; Georgieva, D.; Mekenyan, O.; Cousins, I.T. Evaluation of human pharmaceutical emissions and concentrations in Swedish river basins. Sci. Total Environ. 2016, 572, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2018, 654, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.; Diab, A.; Alsuhaimi, A.; Niazy, E.; Metwally, M.; Amad, M.; Sioud, S.; Dawoud, A. Analysis of some pharmaceuticals in municipal wastewater of Almadinah Almunawarah. Arab. J. Chem. 2017, 10, S719–S729. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Li, C.; Dhangar, K.; Kumar, M. Predicted occurrence, ecotoxicological risk and environmentally acquired resistance of antiviral drugs associated with COVID-19 in environmental waters. Sci. Total Environ. 2021, 776, 145740. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- European Union. Decision (EU) 2022/1307 of 22 July 2022 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2022, 197, 117–120. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/?uri=CELEX:32022D1307 (accessed on 16 November 2022).
- European Union. Decision (EU) 2020/1161 of 4 August 2020 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Communities 2020, 257, 32–35. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/?uri=CELEX%3A32020D1161 (accessed on 16 November 2022).
- Gilardoni, A. (Ed.) The Italian Water Industry: Cases of Excellence. Circular Economy and WWTPs: Water Reuse and Biogas Production Chapter; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Smol, M.; Koneczna, R. Economic Indicators in Water and Wastewater Sector Contributing to a Circular Economy (CE). Resources 2021, 10, 129. [Google Scholar] [CrossRef]
- Carter, L.J.; Chefetz, B.; Abdeen, Z.; Boxall, A.B. Emerging investigator series: Towards a framework for establishing the impacts of pharmaceuticals in wastewater irrigation systems on agro-ecosystems and human health. Environ. Sci. Process. Impacts 2019, 21, 605–622. [Google Scholar] [CrossRef]
- Ponce-Robles, L.; Benelhadj, L.; García-García, A.J.; Pedrero-Salcedo, F.; Nortes-Tortosa, P.A.; Albacete, J.; Alarcón, J.J. Risk assessment for uptake and accumulation of pharmaceuticals by baby leaf lettuce irrigated with reclaimed water under commercial agricultural activities. J. Environ. Manag. 2022, 324, 116321. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on minimum requirements for water reuse. Off. J. Eur. Union 2022, 177, 32–55. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741&from=EN (accessed on 17 November 2022).
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Maniakova, G.; Salmerón, I.; Aliste, M.; Polo-López, M.I.; Oller, I.; Malato, S.; Rizzo, L. Solar photo-Fenton at circumneutral pH using Fe (III)-EDDS compared to ozonation for tertiary treatment of urban wastewater: Contaminants of emerging concern removal and toxicity assessment. Chem. Eng. J. 2022, 431, 133474. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.; Silva, A.M. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.C.; Florencio, M.H. Photocatalytic degradation of amitriptyline, trazodone and venlafaxine using modified cobalt-titanate nanowires under UV–Vis radiation: Transformation products and in silico toxicity. Chem. Eng. J. 2019, 373, 1338–1347. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wang, D.; Ni, H.; Wu, M. Tuning Lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, M.; Xu, L.; Wang, S.; Yang, T.; Wu, M.; Lu, W.; Li, Y.; Yu, D. Unraveling timescale-dependent Fe-MOFs crystal evolution for catalytic ozonation reactivity modulation. J. Hazard. Mater. 2022, 431, 128575. [Google Scholar] [CrossRef]
- Fan, W.; An, W.G.; Huo, M.X.; Yang, W.; Zhu, S.Y.; Lin, S.S. Solubilization and stabilization for prolonged reactivity of ozone using micro-nano bubbles and ozone-saturated solvent: A promising enhancement for ozonation. Sep. Purif. Technol. 2020, 238, 116484. [Google Scholar] [CrossRef]
- Hu, L.; Xia, Z. Application of ozone micro-nano-bubbles to groundwater remediation. J. Hazard. Mater. 2018, 342, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. ζ potential of microbubbles in aqueous solutions: Electrical properties of the gas—Water interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef] [PubMed]
- Gurung, A.; Dahl, O.; Jansson, K. The fundamental phenomena of nanobubbles and their behavior in wastewater treatment technologies. Geosyst. Eng. 2016, 19, 133–142. [Google Scholar] [CrossRef]
- Nam, G.; Mohamed, M.M.; Jung, J. Enhanced degradation of benzo [a] pyrene and toxicity reduction by microbubble ozonation. Environ. Technol. 2021, 42, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y.; Hayashi, T. Removal of pharmaceuticals in water by introduction of ozonated microbubbles. Sep. Purif. Technol. 2019, 212, 483–489. [Google Scholar] [CrossRef]
- Dawood, F.K.; Abdulrazzaq, N.N. Direct Oxidation of Antibiotics from Aqueous Solution by Ozonation with Microbubbles. J. Phys. Conf. Ser. 2021, 1973, 012157. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, T.; Wang, Q.; Tian, Y.; Shi, Z.; Smale, N.; Xu, B. Advanced treatment of acrylic fiber manufacturing wastewater with a combined microbubble-ozonation/ultraviolet irradiation process. RSC Adv. 2015, 5, 77601–77609. [Google Scholar] [CrossRef]
- Tsuge, H. Characteristics of microbubbles. Micro- and Nanobubbles Fundamentals and Applications; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2014; Volume 2, pp. 978–981. [Google Scholar]
- Joseph, C.G.; Farm, Y.Y.; Taufiq-Yap, Y.H.; Pang, C.K.; Nga, J.L.; Puma, G.L. Ozonation treatment processes for the remediation of detergent wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 106099. [Google Scholar] [CrossRef]
- Kokkoli, A.; Agerholm, N.; Andersen, H.R.; Kaarsholm, K.M. Synergy between ozonation and GAC filtration for chlorinated ethenes-contaminated groundwater treatment. J. Water Process Eng. 2021, 44, 102356. [Google Scholar] [CrossRef]
- Bui, T.T.; Han, M. Decolorization of dark green Rit dye using positively charged nanobubbles technologies. Sep. Purif. Technol. 2020, 233, 116034. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.G.; Hey, G.; Vega, S.R.; Spiliotopoulou, A.; Fick, J.; Tysklind, M.; la Cour Jansen, J.; Andersen, H.R. Required ozone doses for removing pharmaceuticals from wastewater effluents. Sci. Total Environ. 2013, 456, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Seridou, P.; Kalogerakis, N. Disinfection applications of ozone micro-and nanobubbles. Environ. Sci. Nano 2021, 8, 3493–3510. [Google Scholar] [CrossRef]
- Gardoni, D.; Vailati, A.; Canziani, R. Decay of ozone in water: A review. Ozone Sci. Eng. 2012, 34, 233–242. [Google Scholar] [CrossRef]
- Rodsong, P.; Tirawanichakul, S.; Tirawanichakul, Y. Disinfection of E. coli and Removal of Pesticide Residues on Fresh Chili by Micro-bubble Plasma Ozonation. Plasma Phys. Membr. Technol. 2021, 9, 1–16. [Google Scholar]
- Sumikura, M.; Hidaka, M.; Murakami, H.; Nobutomo, Y.; Murakami, T. Ozone micro-bubble disinfection method for wastewater reuse system. Water Sci. Technol. 2007, 56, 53–61. [Google Scholar] [CrossRef]
- Beltran, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Hu, L. Treatment of organics contaminated wastewater by ozone micro-nano-bubbles. Water 2018, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.; Spiliotopoulou, A.; Chhetri, R.K.; Ooi, G.T.; Kaarsholm, K.M.; Sundmark, K.; Florian, B.; Kragelund, C.; Bester, K.; Andersen, H.R. Removal of pharmaceuticals, toxicity and natural fluorescence through the ozonation of biologically-treated hospital wastewater, with further polishing via a suspended biofilm. Chem. Eng. J. 2019, 359, 321–330. [Google Scholar] [CrossRef]
- Tay, K.S.; Madehi, N. Ozonation of ofloxacin in water: By-products, degradation pathway and ecotoxicity assessment. Sci. Total Environ. 2015, 520, 23–31. [Google Scholar] [CrossRef]
- Kıdak, R.; Doğan, Ş. Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrason. Sonochem. 2018, 40, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Luiz, D.B.; Genena, A.K.; Virmond, E.; José, H.J.; Moreira, R.F.; Gebhardt, W.; Schröder, H.F. Identification of degradation products of erythromycin A arising from ozone and advanced oxidation process treatment. Water Environ. Res. 2010, 82, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Watts, M.J.; Yeh, D.; Esposito, G.; van Hullebusch, E.D. The efficacy of ozone/BAC treatment on non-steroidal anti-inflammatory drug removal from drinking water and surface water. Ozone Sci. Eng. 2015, 37, 343–356. [Google Scholar] [CrossRef]
- Gulde, R.; Rutsch, M.; Clerc, B.; Schollée, J.E.; von Gunten, U.; McArdell, C.S. Formation of transformation products during ozonation of secondary wastewater effluent and their fate in post-treatment: From laboratory-to full-scale. Water Res. 2021, 200, 117200. [Google Scholar] [CrossRef]
- Hübner, U.; Seiwert, B.; Reemtsma, T.; Jekel, M. Ozonation products of carbamazepine and their removal from secondary effluents by soil aquifer treatment–indications from column experiments. Water Res. 2014, 49, 34–43. [Google Scholar] [CrossRef]
- Kråkström, M.; Saeid, S.; Tolvanen, P.; Kumar, N.; Salmi, T.; Kronberg, L.; Eklund, P. Ozonation of carbamazepine and its main transformation products: Product determination and reaction mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 23258–23269. [Google Scholar] [CrossRef] [PubMed]
- Sein, M.M.; Zedda, M.; Tuerk, J.; Schmidt, T.C.; Golloch, A.; Von Sonntag, C. Oxidation of diclofenac with ozone in aqueous solution. Environ. Sci. Technol. 2008, 42, 6656–6662. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.K.; Ansari, A.J.; Nghiem, L.D.; Price, W.E. New transformation products from ozonation and photolysis of diclofenac in the aqueous phase. Process Saf. Environ. Prot. 2022, 157, 106–114. [Google Scholar] [CrossRef]
- Rodayan, A.; Roy, R.; Yargeau, V. Oxidation products of sulfamethoxazole in ozonated secondary effluent. J. Hazard. Mater. 2010, 177, 237–243. [Google Scholar] [CrossRef]
- Khan, M.H.; Bae, H.; Jung, J.Y. Tetracycline degradation by ozonation in the aqueous phase: Proposed degradation intermediates and pathway. J. Hazard. Mater. 2010, 181, 659–665. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Pinto, G.; Pollio, A. Carbamazepine in water: Persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res. 2002, 36, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, W.; Schröder, H.F. Liquid chromatography–(tandem) mass spectrometry for the follow-up of the elimination of persistent pharmaceuticals during wastewater treatment applying biological wastewater treatment and advanced oxidation. J. Chromatogr. A 2007, 1160, 34–43. [Google Scholar] [CrossRef] [PubMed]
- BOE 2007 Royal Decree 1620/2007, 294, 50639–50661. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2007-21092 (accessed on 30 November 2022).
- Furuichi, A.; Arakawa, S.; Mano, Y.; Morita, I.; Tachikawa, N.; Yamada, Y.; Kasugai, S. Comparative analysis of efficacy of ozone nano bubble water (NBW3) with established antimicrobials. Bactericidal efficacy and cellular response. An in vitro study. J. Oral Tissue Eng. 2013, 10, 131–141. [Google Scholar]
- Cruz, R.; Flores, J.V. Reduction of coliforms presents in domestic residual waters by air-ozone micro-nanobubbles in Carhuaz city, Perú. J. Nanotechnol. 2017, 1, 9–17. [Google Scholar] [CrossRef]
- Vivar, M.; Fuentes, M.; Torres, J.; Rodrigo, M.J. Solar disinfection as a direct tertiary treatment of a wastewater plant using a photochemical-photovoltaic hybrid system. J. Water Process Eng. 2021, 42, 102196. [Google Scholar] [CrossRef]
- Rojas-Valencia, M.N. Research on ozone application as disinfectant and action mechanisms on wastewater microorganisms. Virus 2011, 3, 4. [Google Scholar]
- Ternes, T.A.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Chuajedton, A.; Uthaibutra, J.; Pengphol, S.; Whangchai, K. Inactivation of Escherichia coli O157: H7 by treatment with different temperatures of micro-bubbles ozone containing water. Int. Food Res. J. 2017, 24, 1006–1010. [Google Scholar]
- Chuajedton, A.; Aoyagi, H.; Uthaibutra, J.; Whangchai, K. Effect of Micro-bubbles Ozone for Inactivation of Escherichia coli O157: H7 on Fresh-cut Pineapple cv. Phu Lae. Asian J. Appl. Sci. 2016, 4, 1. [Google Scholar]
- Seki, M.; Ishikawa, T.; Terada, H.; Nashimoto, M. Microbicidal effects of stored aqueous ozone solution generated by nano-bubble technology. Vivo 2017, 31, 579–583. [Google Scholar]
- Mehrjouei, M.; Müller, S.; Möller, D. Catalytic and photocatalytic ozonation of tert-butyl alcohol in water by means of falling film reactor: Kinetic and cost–effectiveness study. Chem. Eng. J. 2014, 248, 184–190. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric-and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Liu, Z.; Demeestere, K.; Van Hulle, S. Comparison and performance assessment of ozone-based AOPs in view of trace organic contaminants abatement in water and wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105599. [Google Scholar] [CrossRef]
- Daneshvar, N.; Aleboyeh, A.; Khataee, A.R. The evaluation of electrical energy per order (EEo) for photooxidative decolorization of four textile dye solutions by the kinetic model. Chemosphere 2005, 59, 761–767. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Ponce-Robles, L.; Masdemont-Hernández, B.; Munuera-Pérez, T.; Pagán-Muñoz, A.; Lara-Guillén, A.J.; García-García, A.J.; Pedrero-Salcedo, F.; Nortes-Tortosa, P.A.; Alarcón-Cabañero, J.J. WWTP effluent quality improvement for agricultural reuse using an autonomous prototype. Water 2020, 12, 2240. [Google Scholar] [CrossRef]
- Race, M.; Ferraro, A.; Galdiero, E.; Guida, M.; Núñez-Delgado, A.; Pirozzi, F.; Siciliano, A.; Fabbricino, M. Current emerging SARS-CoV-2 pandemic: Potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020, 188, 109808. [Google Scholar] [CrossRef]
- Stingl, J.C. Antidepressant drug treatment protecting from COVID-19: One more piece in the repurposing puzzle. BJPsych Open 2022, 8, e20. [Google Scholar] [CrossRef]
- Resolution of 19 June 2020, of the Spanish Agency for Medicines and Medical Devices, Establishing the List of Medicines Considered Essential in the Management of the Health Crisis Caused by COVID-19, Pursuant to Article 19.1 of Royal Decree-Law 21/2020 of 9 June, on Urgent Prevention, Containment and Coordination Measures to Address the Health Crisis Caused by COVID-19. Available online: https://www.boe.es/boe/dias/2020/06/20/pdfs/BOE-A-2020-6474.pdf (accessed on 17 November 2022).
- PubChem, National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 17 November 2022).
- Network of Reference Laboratories, Research Centres and Related Organisations for Monitoring of Emerging Environmental Substances. Available online: https://www.norman-network.net (accessed on 17 November 2022).
- Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; Rice, E.W., Ed.; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Wef, A.A. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- ISO 9308-1: 2014; International Standards Organisation 9308-1-Water Quality-Determination and Counting of Escherichia Coli and Coliform Bacteria-Part 1: Membrane Filtration Method for Low Bacterial Ground Water. Turkish Standards Institute: Ankara, Turkey, 2014.
- ISO 14189; Water Quality—Enumeration of Clostridium Perfringens—Method Using Membrane Filtration. International Standards Organisation (ISO): Geneva, Switzerland, 2013.
- Martinez-Alcala, I.; Guillén-Navarro, J.M.; Fernández-López, C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 2017, 316, 332–340. [Google Scholar] [CrossRef]
- Cantwell, H. Blanks in Method Validation—Supplement to Eurachem Guide the Fitness for Purpose of Analytical Methods; Eurachem: Middlesex, UK, 2019. [Google Scholar]






| Drug Type | Formula | Molecular Weight (g/mol) | Log Kow | pKa | |
|---|---|---|---|---|---|
| Acetaminophen (ACT) | Analgesic | C37H67NO13 | 151.16 | 3.06 | 8.9 |
| Amoxicillin (AMX) | Antibacterial | C16H19N3O5S | 365.4 | 0.87 | 2.6 |
| Carbamazepine (CBZ) | Anticonvulsant | C15H12N2O | 236.3 | 2.45 | 15.9, −3.8 |
| Chloroquine (CHL) | Antimalarial | C18H26ClN3 | 319.9 | 4.63 | 10.1 |
| Diclofenac (DCF) | Anti-inflammatory | C14H11Cl2NO2 | 296.1 | 4.51 | 3.9 |
| Erythromycin (ERY) | Antibacterial | C37H67NO13 | 733.9 | 3.06 | 8.8 |
| Haloperidol (HLP) | Antipsychotic | C21H23ClFNO2 | 375.9 | 4.30 | 8.65 |
| Ketoprofen (KTP) | Anti-inflammatory | C16H14O3 | 254.3 | - | 3.98 |
| Naproxen (NPX) | Anti-inflammatory | C14H14O3 | 230.26 | 3.18 | 4.18 |
| Sulfamethoxazole (SMX) | Anticonvulsant | C10H11N3O3S | 253.28 | 0.89 | pKa1 = 1.6 pKa2 = 5.7 |
| Tetracycline (TCL) | Antibacterial | C22H24N2O8 | 444.4 | −1.37 | 7.68, 3.3 |
| Trazodone (TRZ) | Antidepressant | C19H22ClN5O | 371.9 | 3.21 | 6.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce-Robles, L.; Pagán-Muñoz, A.; Lara-Guillén, A.J.; Masdemont-Hernández, B.; Munuera-Pérez, T.; Nortes-Tortosa, P.A.; Alarcón-Cabañero, J.J. Full-Scale O3/Micro-Nano Bubbles System Based Advanced Oxidation as Alternative Tertiary Treatment in WWTP Effluents. Catalysts 2023, 13, 188. https://doi.org/10.3390/catal13010188
Ponce-Robles L, Pagán-Muñoz A, Lara-Guillén AJ, Masdemont-Hernández B, Munuera-Pérez T, Nortes-Tortosa PA, Alarcón-Cabañero JJ. Full-Scale O3/Micro-Nano Bubbles System Based Advanced Oxidation as Alternative Tertiary Treatment in WWTP Effluents. Catalysts. 2023; 13(1):188. https://doi.org/10.3390/catal13010188
Chicago/Turabian StylePonce-Robles, Laura, Aránzazu Pagán-Muñoz, Andrés Jesús Lara-Guillén, Beatriz Masdemont-Hernández, Teresa Munuera-Pérez, Pedro Antonio Nortes-Tortosa, and Juan José Alarcón-Cabañero. 2023. "Full-Scale O3/Micro-Nano Bubbles System Based Advanced Oxidation as Alternative Tertiary Treatment in WWTP Effluents" Catalysts 13, no. 1: 188. https://doi.org/10.3390/catal13010188
APA StylePonce-Robles, L., Pagán-Muñoz, A., Lara-Guillén, A. J., Masdemont-Hernández, B., Munuera-Pérez, T., Nortes-Tortosa, P. A., & Alarcón-Cabañero, J. J. (2023). Full-Scale O3/Micro-Nano Bubbles System Based Advanced Oxidation as Alternative Tertiary Treatment in WWTP Effluents. Catalysts, 13(1), 188. https://doi.org/10.3390/catal13010188