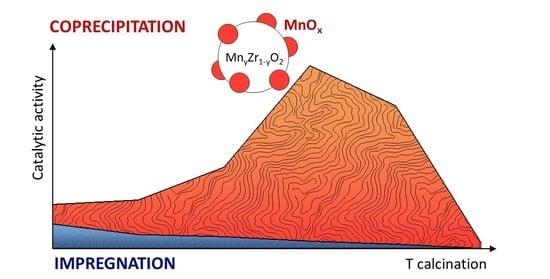
The Catalytic Performance of CO Oxidation over MnOx-ZrO2 Catalysts: The Role of Synthetic Routes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Catalytic Activity in CO Oxidation for the IM and COP Series
2.2. Structural and Microstructural Properties
2.3. TEM Study (Morphology, Composition, and Distribution)
2.4. XANES Study
2.5. Results of the TPR-H2 Analysis
2.6. XPS Study
2.7. Factors Determining the Catalytic Activity
3. Experimental Procedure
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Sun, H.; Park, S.-J. Recent Advances in MnOx/CeO2-Based Ternary Composites for Selective Catalytic Reduction of NOx by NH3: A Review. Catalysts 2021, 11, 1519. [Google Scholar] [CrossRef]
- Xu, H.; Yan, N.; Qu, Z.; Liu, W.; Mei, J.; Huang, W.; Zhao, S. Gaseous Heterogeneous Catalytic Reactions over Mn-Based Oxides for Environmental Applications: A Critical Review. Environ. Sci. Technol. 2017, 51, 8879–8892. [Google Scholar] [CrossRef] [PubMed]
- Bratan, V.; Vasile, A.; Chesler, P.; Hornoiu, C. Insights into the Redox and Structural Properties of CoOx and MnOx: Fundamental Factors Affecting the Catalytic Performance in the Oxidation Process of VOCs. Catalysts 2022, 12, 1134. [Google Scholar] [CrossRef]
- Arena, F.; Torre, T.; Raimondo, C.; Parmaliana, A. Structure and redox properties of bulk and supported manganese oxide catalysts. Phys. Chem. Chem. Phys. 2001, 3, 1911–1917. [Google Scholar] [CrossRef]
- Trawczyński, J.; Bielak, B.; Miśta, W. Oxidation of ethanol over supported manganese catalysts—Effect of the carrier. Appl. Catal. B Environ. 2005, 55, 277–285. [Google Scholar] [CrossRef]
- Qu, Y.; Zhu, S.; Zhang, L.; Sun, S.; Dong, X.; Guo, L. Low-temperature catalytic combustion of benzene over Zr–Mn mixed oxides synthesized by redox-precipitation method. J. Mater. Sci. 2021, 56, 13540–13555. [Google Scholar] [CrossRef]
- Fernández López, E.; Sánchez Escribano, V.; Resini, C.; Gallardo-Amores, J.M.; Busca, G. A study of coprecipitated Mn–Zr oxides and their behaviour as oxidation catalysts. Appl. Catal. B Environ. 2001, 29, 251–261. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Uphade, B.S.; Pataskar, S.G. Low temperature complete combustion of dilute methane over Mn-doped ZrO2 catalysts: Factors influencing the reactivity of lattice oxygen and methane combustion activity of the catalyst. Appl. Catal. A Gen. 2002, 227, 29–41. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Jing, M.; Song, W.; Liu, J.; Ge, M. Defective MnxZr1−xO2 Solid Solution for the Catalytic Oxidation of Toluene: Insights into the Oxygen Vacancy Contribution. ACS Appl. Mater. Interfaces 2019, 11, 730–739. [Google Scholar] [CrossRef]
- Kaplin, I.Y.; Lokteva, E.S.; Golubina, E.V.; Shishova, V.V.; Maslakov, K.I.; Fionov, A.V.; Isaikina, O.Y.; Lunin, V.V. Efficiency of manganese modified CTAB-templated ceria-zirconia catalysts in total CO oxidation. Appl. Surf. Sci. 2019, 485, 432–440. [Google Scholar] [CrossRef]
- Gutiérrez-Ortiz, J.I.; de Rivas, B.; López-Fonseca, R.; Martín, S.; González-Velasco, J.R. Structure of Mn-Zr mixed oxides catalysts and their catalytic performance in the gas-phase oxidation of chlorocarbons. Chemosphere 2007, 68, 1004–1012. [Google Scholar] [CrossRef]
- Döbber, D.; Kießling, D.; Schmitz, W.; Wendt, G. MnOx/ZrO2 catalysts for the total oxidation of methane and chloromethane. Appl. Catal. B Environ. 2004, 52, 135–143. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Glyzdova, D.V.; Konovalova, V.P.; Saraev, A.A.; Aydakov, E.E.; Bulavchenko, O.A. Effect of Calcination Temperature on the Properties of Mn–Zr–Ce Catalysts in the Oxidation of Carbon Monoxide. Kinet. Catal. 2022, 63, 431–439. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Figueroa, S.J.A.; Requejo, F.G.; Lede, E.J.; Lamaita, L.; Peluso, M.A.; Sambeth, J.E. XANES study of electronic and structural nature of Mn-sites in manganese oxides with catalytic properties. Catal. Today 2005, 107–108, 849–855. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef] [Green Version]
- Jiao, F.; Frei, H. Nanostructured manganese oxide clusters supported on mesoporous silica as efficient oxygen-evolving catalysts. Chem. Commun. 2010, 46, 2920–2922. [Google Scholar] [CrossRef]
- Pankin, I.A.; Kravtsova, A.N.; Polozhentsev, O.E.; Budnyk, A.P.; Tsaturyan, A.A.; Bugaev, A.L.; Trigub, A.L.; Soldatov, A.V. X-ray spectroscopic diagnostics of the structure of quantum dots based on zinc and manganese sulfides and oxides. J. Struct. Chem. 2017, 58, 1633–1640. [Google Scholar] [CrossRef]
- Ramallo-López, J.M.; Lede, E.J.; Requejo, F.G.; Rodriguez, J.A.; Kim, J.-Y.; Rosas-Salas, R.; Domínguez, J.M. XANES Characterization of Extremely Nanosized Metal-Carbonyl Subspecies (Me = Cr, Mn, Fe, and Co) Confined into the Mesopores of MCM-41 Materials. J. Phys. Chem. B 2004, 108, 20005–20010. [Google Scholar] [CrossRef]
- Ressler, T.; Brock, S.L.; Wong, J.; Suib, S.L. Multiple-Scattering EXAFS Analysis of Tetraalkylammonium Manganese Oxide Colloids. J. Phys. Chem. B 1999, 103, 6407–6420. [Google Scholar] [CrossRef]
- Stobbe, E.R.; De Boer, B.A.; Geus, J.W. The reduction and oxidation behaviour of manganese oxides. Catal. Today 1999, 47, 161–167. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Venediktova, O.S.; Afonasenko, T.N.; Tsyrul’Nikov, P.G.; Saraev, A.A.; Kaichev, V.V.; Tsybulya, S.V. Nonstoichiometric oxygen in Mn-Ga-O spinels: Reduction features of the oxides and their catalytic activity. RSC Adv. 2018, 8, 11598–11607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Song, L.; Tang, S.; Liu, C.; Yue, H.; Liang, B. Insight into the synergism between MnO2 and acid sites over Mn–SiO2@TiO2 nano-cups for low-temperature selective catalytic reduction of NO with NH3. RSC Adv. 2018, 8, 1979–1986. [Google Scholar] [CrossRef] [Green Version]
- Kulchakovskaya, E.V.; Dotsenko, S.S.; Liotta, L.F.; La Parola, V.; Galanov, S.I.; Sidorova, O.I.; Vodyankina, O.V. Synergistic Effect in Ag/Fe–MnO2 Catalysts for Ethanol Oxidation. Catalysts 2022, 12, 872. [Google Scholar]
- Koh, D.J.; Chung, J.S.; Kim, Y.G.; Lee, J.S.; Nam, I.S.; Heup Moon, S. Structure of MnZr mixed oxide catalysts and their catalytic properties in the CO hydrogenation reaction. J. Catal. 1992, 138, 630–639. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Afonasenko, T.N.; Tsyrul’Nikov, P.G.; Tsybulya, S.V. Effect of heat treatment conditions on the structure and catalytic properties of MnOx/Al2O3 in the reaction of CO oxidation. Appl. Catal. A Gen. 2013, 459, 73–80. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Glyzdova, D.V.; Yurpalov, V.L.; Konovalova, V.P.; Rogov, V.A.; Gerasimov, E.Y.; Bulavchenko, O.A. The Study of Thermal Stability of Mn-Zr-Ce, Mn-Ce and Mn-Zr Oxide Catalysts for CO Oxidation. Materials 2022, 15, 7553. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Vinokurov, Z.S.; Afonasenko, T.N.; Tsyrul’Nikov, P.G.; Tsybulya, S.V.; Saraev, A.A.; Kaichev, V.V. Reduction of mixed Mn-Zr oxides: In situ XPS and XRD studies. Dalton Trans. 2015, 44, 15499–15507. [Google Scholar] [CrossRef] [Green Version]
- Li, W.B.; Chu, W.B.; Zhuang, M.; Hua, J. Catalytic oxidation of toluene on Mn-containing mixed oxides prepared in reverse microemulsions. Catal. Today 2004, 93–95, 205–209. [Google Scholar] [CrossRef]
- Castro, V.D.; Polzonetti, G. XPS study of MnO oxidation. J. Electron Spectrosc. Relat. Phenom. 1989, 48, 117–123. [Google Scholar]
- Han, Y.-F.; Chen, F.; Zhong, Z.; Ramesh, K.; Chen, L.; Widjaja, E. Controlled Synthesis, Characterization, and Catalytic Properties of Mn2O3 and Mn3O4 Nanoparticles Supported on Mesoporous Silica SBA-15. J. Phys. Chem. B 2006, 110, 24450–24456. [Google Scholar] [CrossRef]
- Feng, X.; Cox, D.F. Na Deposition on MnO(100). Surf. Sci. 2016, 645, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Oku, M.; Hirokawa, K.; Ikeda, S. X-ray photoelectron spectroscopy of manganese—oxygen systems. J. Electron Spectrosc. Relat. Phenom. 1975, 7, 465–473. [Google Scholar] [CrossRef]
- Regan, E.; Groutso, T.; Metson, J.B.; Steiner, R.; Ammundsen, B.; Hassell, D.; Pickering, P. Surface and bulk composition of lithium manganese oxides. Surf. Interface Anal. 1999, 27, 1064–1068. [Google Scholar] [CrossRef]
- Bondi, J.F.; Oyler, K.D.; Ke, X.; Schiffer, P.; Schaak, R.E. Chemical synthesis of air-stable manganese nanoparticles. J. Am. Chem. Soc. 2009, 131, 9144–9145. [Google Scholar] [CrossRef]
- Han, Y.-F.; Chen, L.; Ramesh, K.; Zhong, Z.; Chen, F.; Chin, J.; Mook, H. Coral-like nanostructured α-Mn2O3 nanaocrystals for catalytic combustion of methane: Part, I. Preparation and characterization. Catal. Today 2008, 131, 35–41. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Zhang, G.; Zheng, J.; Wang, T.; Liu, X.; Shu, C.; Jiang, L.; Wang, C. Enhanced electrocatalytic performance for methanol oxidation of Pt nanoparticles on Mn3O4-modified multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2012, 37, 11167–11175. [Google Scholar] [CrossRef]
- Ramesh, K.; Chen, L.; Chen, F.; Liu, Y.; Wang, Z.; Han, Y.-F. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts. Catal. Today 2008, 131, 477–482. [Google Scholar] [CrossRef]
- Kostowskyj, M.A.; Kirk, D.W.; Thorpe, S.J. Ag and Ag–Mn nanowire catalysts for alkaline fuel cells. Int. J. Hydrogen Energy 2010, 35, 5666–5672. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, W.; Li, Y.; Chen, Q.; Zhan, F. Nitrogen-doped graphene aerogel-supported spinel CoMn2O4 nanoparticles as an efficient catalyst for oxygen reduction reaction. J. Power Sources 2015, 299, 492–500. [Google Scholar] [CrossRef]
- Kong, W.; Gao, B.; Jiang, C.; Chang, A. Influence of the oxygen pressure on the preferred orientation and optical properties of the pulsed-laser deposited Mn1.56Co0.96Ni0.48O4 ±δ thin films. J. Alloys Compd. 2015, 650, 305–310. [Google Scholar] [CrossRef]
- Jadhav, P.R.; Suryawanshi, M.P.; Dalavi, D.S.; Patil, D.S.; Jo, E.A.; Kolekar, S.S.; Wali, A.A.; Karanjkar, M.M.; Kim, J.-H.; Patil, P.S. Design and electro-synthesis of 3-D nanofibers of MnO2 thin films and their application in high performance supercapacitor. Electrochim. Acta 2015, 176, 523–532. [Google Scholar] [CrossRef]
- Guittet, M.J.; Crocombette, J.P.; Gautier-Soyer, M. Bonding and XPS chemical shifts in ZrSiO4 versus SiO2 and ZrO2: Charge transfer and electrostatic effects. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 63, 1251171–1251177. [Google Scholar] [CrossRef]
- Jones, D.J.; Jiménez-Jiménez, J.; Jiménez-López, A.; Maireles-Torres, P.; Olivera-Pastor, P.; Rodriguez-Castellón, E.; Rozière, J. Surface characterisation of zirconium-doped mesoporous silica. Chem. Commun. 1997, 5, 431–432. [Google Scholar] [CrossRef]
- Miyazaki, S. Characterization of high-k gate dielectric/silicon interfaces. Appl. Surf. Sci. 2002, 190, 66–74. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Asami, K.; Ito, S.; Yashima, M.; Sugimoto, T. XPS study of the phase transition in pure zirconium oxide nanocrystallites. Appl. Surf. Sci. 2005, 252, 1651–1656. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Bulavchenko, O.A.; Gulyaeva, T.I.; Tsybulya, S.V.; Tsyrul’nikov, P.G. Effect of the Calcination Temperature and Composition of the MnOx–ZrO2 System on Its Structure and Catalytic Properties in a Reaction of Carbon Monoxide Oxidation. Kinet. Catal. 2018, 59, 104–111. [Google Scholar] [CrossRef]
- Zeng, K.; Li, X.; Wang, C.; Wang, Z.; Guo, P.; Yu, J.; Zhang, C.; Zhao, X.S. Three-dimensionally macroporous MnZrOx catalysts for propane combustion: Synergistic structure and doping effects on physicochemical and catalytic properties. J. Colloid Interface Sci. 2020, 572, 281–296. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]







| Calcination Temperature, °C | COP | IM | ||||||
|---|---|---|---|---|---|---|---|---|
| Phase Composition, wt % | Crystallite Size, Å | Lattice Parameters of ZrO2 *, Å (V/N, Å3) | Microstrains in ZrO2 * | SBET, m2/g | Phase Composition, wt % | Crystallite Size, Å | SBET, m2/g | |
| 400 | amorphous | - | - | - | 331 | 77% ZrO2 (mon) 15% ZrO2 (tetr) 8% MnO2 | 215 140 190 | 54 |
| 500 | amorphous | - | - | - | 232 | 71% ZrO2 (mon) 13% ZrO2 (tetr) 16% Mn2O3 | 210 120 320 | 51 |
| 600 | ZrO2 * (cub) | 270 | 5.078 (16.37) | 0.006 | 58 | 69% ZrO2 (mon) 11% ZrO2 (tetr) 20% Mn2O3 | 215 120 280 | 43 |
| 650 | ZrO2 * (cub) | 390 | 5.072 (16.31) | 0.008 | 42 | 69% ZrO2 (mon) 12% ZrO2 (tetr) 19% Mn2O3 | 215 130 300 | 35 |
| 700 | ZrO2 * (cub) | 320 | 5.083 (16.41) | 0.007 | 38 | 72% ZrO2 (mon) 8% ZrO2 (tetr) 20% Mn2O3 | 215 150 300 | 27 |
| 800 | 80% ZrO2 (tetr) 13% Mn3O4 7% ZrO2 (mon) | 340 210 120 | a = b = 3.591, c = 5.162 (16.65) | 0.004 | 14 | 72% ZrO2 (mon) 8% ZrO2 (tetr) 20% Mn2O3 | 240 210 310 | 13 |
| IM | COP | |||||||
|---|---|---|---|---|---|---|---|---|
| T, °C | [Mn]/[Zr] | %Mn2+ | %Mn3+ | %Mn4+ | [Mn]/[Zr] | %Mn2+ | %Mn3+ | %Mn4+ |
| 400 | 0.49 | 3 | 45 | 52 | 0.19 | 0 | 46 | 54 |
| 500 | - | - | - | - | 0.20 | 0 | 61 | 39 |
| 600 | 1.25 | 20 | 57 | 23 | 0.27 | 0 | 59 | 41 |
| 650 | 1.15 | 16 | 56 | 28 | 0.29 | 0 | 63 | 37 |
| 700 | 2.41 | 17 | 60 | 23 | 0.31 | 0 | 65 | 35 |
| 800 | 1.13 | 19 | 57 | 24 | 0.35 | 0 | 59 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulavchenko, O.A.; Konovalova, V.P.; Saraev, A.A.; Kremneva, A.M.; Rogov, V.A.; Gerasimov, E.Y.; Afonasenko, T.N. The Catalytic Performance of CO Oxidation over MnOx-ZrO2 Catalysts: The Role of Synthetic Routes. Catalysts 2023, 13, 57. https://doi.org/10.3390/catal13010057
Bulavchenko OA, Konovalova VP, Saraev AA, Kremneva AM, Rogov VA, Gerasimov EY, Afonasenko TN. The Catalytic Performance of CO Oxidation over MnOx-ZrO2 Catalysts: The Role of Synthetic Routes. Catalysts. 2023; 13(1):57. https://doi.org/10.3390/catal13010057
Chicago/Turabian StyleBulavchenko, Olga A., Valeriya P. Konovalova, Andrey A. Saraev, Anna M. Kremneva, Vladimir A. Rogov, Evgeny Yu. Gerasimov, and Tatyana N. Afonasenko. 2023. "The Catalytic Performance of CO Oxidation over MnOx-ZrO2 Catalysts: The Role of Synthetic Routes" Catalysts 13, no. 1: 57. https://doi.org/10.3390/catal13010057
APA StyleBulavchenko, O. A., Konovalova, V. P., Saraev, A. A., Kremneva, A. M., Rogov, V. A., Gerasimov, E. Y., & Afonasenko, T. N. (2023). The Catalytic Performance of CO Oxidation over MnOx-ZrO2 Catalysts: The Role of Synthetic Routes. Catalysts, 13(1), 57. https://doi.org/10.3390/catal13010057