CCl4-TMEDA-CuCl—A Novel Convenient Catalytic System for Dimerization of Terminal Acetylenes in Mild Conditions
Abstract
:1. Introduction
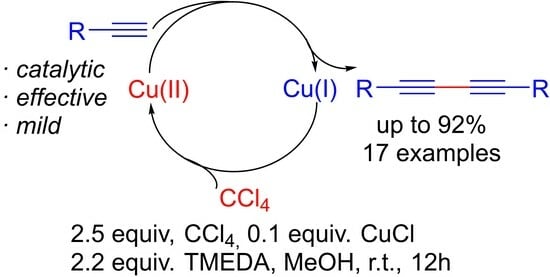
2. Results
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shastin, A.V.; Korotchenko, V.N.; Nenaidenko, V.G.; Balenkova, E.S. A Novel Reaction of Double Carbon-Carbon Bond Formation: Synthesis of 2,2-Dichlorostyrenes. Russ. Chem. Bull. 1999, 48, 2184–2185. [Google Scholar] [CrossRef]
- Shastin, A.V.; Korotchenko, V.N.; Nenajdenko, V.G.; Balenkova, E.S. A Novel Synthetic Approach to Dichlorostyrenes. Tetrahedron 2000, 56, 6557–6563. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Korotchenko, V.N.; Shastin, A.V.; Balenkova, E.S. Catalytic Olefination of Carbonyl Compounds. A New Versatile Method for the Synthesis of Alkenes. Russ. Chem. Bull. 2004, 53, 1034–1064. [Google Scholar] [CrossRef]
- Shixaliyev, N.Q.; Gurbanov, A.V.; Maharramov, A.M.; Mahmudov, K.T.; Kopylovich, M.N.; Martins, L.M.D.R.S.; Muzalevskiy, V.M.; Nenajdenko, V.G.; Pombeiro, A.J.L. Halogen-Bonded Tris(2,4-Bis(Trichloromethyl)-1,3,5-Triazapentadienato)-M(iii) [M = Mn, Fe, Co] Complexes and Their Catalytic Activity in the Peroxidative Oxidation of 1-Phenylethanol to Acetophenone. New J. Chem. 2014, 38, 4807–4815. [Google Scholar] [CrossRef]
- Shastin, A.V.; Korotchenko, V.N.; Nenajdenko, V.G.; Balenkova, E.S. A Novel Synthesis of β,β-Dibromostyrenes. Synthesis 2001, 2001, 2081–2084. [Google Scholar] [CrossRef]
- Motornov, V.A.; Muzalevskiy, V.M.; Tabolin, A.A.; Novikov, R.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Radical Nitration-Debromination of α-Bromo-α-Fluoroalkenes as a Stereoselective Route to Aromatic α-Fluoronitroalkenes—Functionalized Fluorinated Building Blocks for Organic Synthesis. J. Org. Chem. 2017, 82, 5274–5284. [Google Scholar] [CrossRef] [PubMed]
- Shastin, A.V.; Muzalevsky, V.M.; Balenkova, E.S.; Nenajdenko, V.G. Stereoselective Synthesis of 1-Bromo-1-Fluorostyrenes. Mendeleev Commun. 2006, 16, 179–180. [Google Scholar] [CrossRef]
- Goldberg, A.A.; Muzalevskiy, V.M.; Shastin, A.V.; Balenkova, E.S.; Nenajdenko, V.G. Novel Efficient Synthesis of β-Fluoro-β-(Trifluoromethyl)Styrenes. J. Fluor. Chem. 2010, 131, 384–388. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Muzalevskiy, V.M.; Shastin, A.V.; Balenkova, E.S.; Kondrashov, E.V.; Ushakov, I.A.; Rulev, A.Y. Fragmentation of Trifluoromethylated Alkenes and Acetylenes by N,N-Binucleophiles. Synthesis of Imidazolines or Imidazolidines (Oxazolidines) Controlled by Substituent. J. Org. Chem. 2010, 75, 5679–5688. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Shastin, A.V.; Korotchenko, V.N.; Varseev, G.N.; Balenkova, E.S. A Novel Approach to 2-Chloro-2-Fluorostyrenes. Eur. J. Org. Chem. 2003, 2003, 302–308. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M. Synthesis of Heterocyclic Compounds Using the Nenajdenko-Shastin Reaction. Chem. Heterocycl. Compd. 2012, 48, 117–125. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Muzalevskiy, V.M.; Shastin, A.V. Polyfluorinated Ethanes as Versatile Fluorinated C2-Building Blocks for Organic Synthesis. Chem. Rev. 2015, 115, 973–1050. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Balenkova, E.S.; Shastin, A.V.; Magerramov, A.M.; Shikhaliev, N.G.; Nenajdenko, V.G. New Method for the Preparation of 3-Diazo-1,3-Dihydroindol-2-Ones. Russ. Chem. Bull. 2011, 60, 2343–2346. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Shastin, A.V.; Gorbachev, V.M.; Shorunov, S.V.; Muzalevskiy, V.M.; Lukianova, A.I.; Dorovatovskii, P.V.; Khrustalev, V.N. Copper-Catalyzed Transformation of Hydrazones into Halogenated Azabutadienes, Versatile Building Blocks for Organic Synthesis. ACS Catal. 2017, 7, 205–209. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F. Transition Metal Catalyzed Glaser and Glaser-Hay Coupling Reactions: Scope, Classical/Green Methodologies and Synthetic Applications. Synth. Commun. 2020, 50, 3337–3368. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, K.; Wen, C.; Li, Q. Nickel-Catalyzed Cross-Coupling of Organoaluminum Reagents with Alkynylhalides for the Synthesis of Symmetrical and Unsymmetrical Conjugated 1,3-Diynes Derivatives. J. Organomet. Chem. 2020, 906, 121040. [Google Scholar] [CrossRef]
- Matsuda, Y.; Naoe, S.; Oishi, S.; Fujii, N.; Ohno, H. Formal [4+2] Reaction between 1,3-Diynes and Pyrroles: Gold(I)-Catalyzed Indole Synthesis by Double Hydroarylation. Chem.—Eur. J. 2015, 21, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, Y. A Facile Copper(i)-Catalyzed Homocoupling of Terminal Alkynes to 1,3-Diynes with Diaziridinone under Mild Conditions. Org. Biomol. Chem. 2013, 11, 7451. [Google Scholar] [CrossRef] [PubMed]
- Singha, R.; Nandi, S.; Ray, J.K. Bromine-Mediated Cyclization of 1,4-Diaryl Buta-1,3-Diyne to 1,2,3-Tribromo-4-Aryl Naphthalene. Tetrahedron Lett. 2012, 53, 6531–6534. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, X. Synthesis of 2,5-Disubstituted Thiophenes via Metal-Free Sulfur Heterocyclization of 1,3-Diynes with Sodium Hydrosulfide. RSC Adv. 2012, 2, 5488. [Google Scholar] [CrossRef]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthesis of Naturally Occurring Polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Chou, T.-C.; Dong, H.; Tian, Y.; Li, Y.; Danishefsky, S.J. Total Synthesis as a Resource in Drug Discovery: The First In Vivo Evaluation of Panaxytriol and Its Derivatives. J. Org. Chem. 2005, 70, 10375–10380. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Metathesis Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4490–4527. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Diederich, F. Linear Monodisperse π-Conjugated Oligomers: Model Compounds for Polymers and More. Angew. Chem. Int. Ed. 1999, 38, 1350–1377. [Google Scholar] [CrossRef]
- Tour, J.M. Conjugated Macromolecules of Precise Length and Constitution. Organic Synthesis for the Construction of Nanoarchitectures. Chem. Rev. 1996, 96, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. (Eds.) Acetylene Chemistry: Chemistry, Biology and Material Science; Wiley-VCH GmbH KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Gholami, M.; Tykwinski, R.R. Oligomeric and Polymeric Systems with a Cross-Conjugated π-Framework. Chem. Rev. 2006, 106, 4997–5027. [Google Scholar] [CrossRef] [PubMed]
- Nenajdenko, V.G.; Sumerin, V.V.; Chernichenko, K.Y.; Balenkova, E.S. A New Route to Annulated Oligothiophenes. Org. Lett. 2004, 6, 3437–3439. [Google Scholar] [CrossRef]
- Marsden, J.A.; Haley, M.M. Carbon Networks Based on Dehydrobenzoannulenes. 5. Extension of Two-Dimensional Conjugation in Graphdiyne Nanoarchitectures. J. Org. Chem. 2005, 70, 10213–10226. [Google Scholar] [CrossRef]
- Eisler, S.; Slepkov, A.D.; Elliott, E.; Luu, T.; McDonald, R.; Hegmann, F.A.; Tykwinski, R.R. Polyynes as a Model for Carbyne: Synthesis, Physical Properties, and Nonlinear Optical Response. J. Am. Chem. Soc. 2005, 127, 2666–2676. [Google Scholar] [CrossRef]
- Stütz, A. Allylamine Derivatives—A New Class of Active Substances in Antifungal Chemotherapy. Angew. Chem. Int. Ed. Engl. 1987, 26, 320–328. [Google Scholar] [CrossRef]
- Lechner, D.; Stavri, M.; Oluwatuyi, M.; Pereda-Miranda, R.; Gibbons, S. The Anti-Staphylococcal Activity of Angelica Dahurica (Bai Zhi). Phytochemistry 2004, 65, 331–335. [Google Scholar] [CrossRef]
- Schmidt, R.; Thorwirth, R.; Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopf, H. Fast, Ligand- and Solvent-Free Synthesis of 1,4-Substituted Buta-1,3-Diynes by Cu-Catalyzed Homocoupling of Terminal Alkynes in a Ball Mill. Chem.—Eur. J. 2011, 17, 8129–8138. [Google Scholar] [CrossRef]
- Lerch, M.L.; Harper, M.K.; Faulkner, D.J. Brominated Polyacetylenes from the Philippines Sponge Diplastrella sp. J. Nat. Prod. 2003, 66, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.F.; Steinreiber, A.; Orru, R.V.A.; Faber, K. Chemoenzymatic Asymmetric Total Syntheses of Antitumor Agents (3R,9R,10R)- and (3S,9R,10R)-Panaxytriol and (R)- and (S)-Falcarinol from Panax ginseng Using an Enantioconvergent Enzyme-Triggered Cascade Reaction. J. Org. Chem. 2002, 67, 9115–9121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Glaser Coupling. In Name Reactions; Springer International Publishing: Cham, Switzerland, 2014; pp. 282–286. ISBN 978-3-319-03978-7. [Google Scholar]
- Sindhu, K.S.; Anilkumar, G. Recent Advances and Applications of Glaser Coupling Employing Greener Protocols. RSC Adv. 2014, 4, 27867–27887. [Google Scholar] [CrossRef]
- Jover, J.; Spuhler, P.; Zhao, L.; McArdle, C.; Maseras, F. Toward a Mechanistic Understanding of Oxidative Homocoupling: The Glaser–Hay Reaction. Catal. Sci. Technol. 2014, 4, 4200–4209. [Google Scholar] [CrossRef]
- Glaser, C. Beiträge Zur Kenntniss Des Acetenylbenzols. Berichte Dtsch. Chem. Ges. 1869, 2, 422–424. [Google Scholar] [CrossRef]
- Eglinton, G.; Galbraith, A.R. Cyclic dyines. Chem. Ind. 1956, 28, 736–737. [Google Scholar]
- Eglinton, G.; Galbraith, A.R. Macrocyclic Acetylenic Compounds. Part I. Cyclotetradeca-1:3-Diyne and Related Compounds. J. Chem. Soc. Resumed 1959, 889–896. [Google Scholar] [CrossRef]
- Hay, A. Communications- Oxidative Coupling of Acetylenes. J. Org. Chem. 1960, 25, 1275–1276. [Google Scholar] [CrossRef]
- Hay, A.S. Oxidative Coupling of Acetylenes. II. J. Org. Chem. 1962, 27, 3320–3321. [Google Scholar] [CrossRef]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef]
- Yin, W.; He, C.; Chen, M.; Zhang, H.; Lei, A. Nickel-Catalyzed Oxidative Coupling Reactions of Two Different Terminal Alkynes Using O2 as the Oxidant at Room Temperature: Facile Syntheses of Unsymmetric 1,3-Diynes. Org. Lett. 2009, 11, 709–712. [Google Scholar] [CrossRef]
- Bedard, A.-C.; Collins, S.K. Phase Separation As a Strategy Toward Controlling Dilution Effects in Macrocyclic Glaser-Hay Couplings. J. Am. Chem. Soc. 2011, 133, 19976–19981. [Google Scholar] [CrossRef]
- Crowley, J.D.; Goldup, S.M.; Gowans, N.D.; Leigh, D.A.; Ronaldson, V.E.; Slawin, A.M.Z. An Unusual Nickel−Copper-Mediated Alkyne Homocoupling Reaction for the Active-Template Synthesis of [2]Rotaxanes. J. Am. Chem. Soc. 2010, 132, 6243–6248. [Google Scholar] [CrossRef] [PubMed]
- Muesmann, T.W.T.; Wickleder, M.S.; Christoffers, J. Preparation of linear aromatic disulfonic acids: New linker molecules for metal-organic frameworks. Synthesis 2011, 17, 2775–2780. [Google Scholar] [CrossRef]
- Meng, X.; Li, C.; Han, B.; Wang, T.; Chen, B. Iron/copper promoted oxidative homo-coupling reaction of terminal alkynes using air as the oxidant. Tetrahedron 2010, 66, 4029–4031. [Google Scholar] [CrossRef]
- Li, J.-H.; Liang, Y.; Xie, Y.-X. Efficient Palladium-Catalyzed Homocoupling Reaction and Sonogashira Cross-Coupling Reaction of Terminal Alkynes under Aerobic Conditions. J. Org. Chem. 2005, 70, 4393–4396. [Google Scholar] [CrossRef] [PubMed]
- Batsanov, A.S.; Collings, J.C.; Fairlamb, I.J.S.; Holland, J.P.; Howard, J.A.K.; Lin, Z.; Marder, T.B.; Parsons, A.C.; Ward, R.M.; Zhu, J. Requirement for an Oxidant in Pd/Cu Co-Catalyzed Terminal Alkyne Homocoupling To Give Symmetrical 1,4-Disubstituted 1,3-Diynes. J. Org. Chem. 2005, 70, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Merkul, E.; Urselmann, D.; Müller, T.J.J. Consecutive One-Pot Sonogashira–Glaser Coupling Sequence—Direct Preparation of Symmetrical Diynes by Sequential Pd/Cu Catalysis. Eur. J. Org. Chem. 2011, 2011, 238–242. [Google Scholar] [CrossRef]
- Hoshi, M.; Okimoto, M.; Nakamura, S.; Takahashi, S. Homo- and Heterocoupling of Terminal Conjugated Enynes: One-Pot Synthesis of Alka-1,7-diene-3,5-diynes and Alk-1-ene-3,5-diynes via Two Types of Coupling Reaction. Synthesis 2011, 23, 3839–3847. [Google Scholar] [CrossRef]
- Oishi, T.; Katayama, T.; Yamaguchi, K.; Mizuno, N. Heterogeneously Catalyzed Efficient Alkyne–Alkyne Homocoupling by Supported Copper Hydroxide on Titanium Oxide. Chem. Eur. J. 2009, 15, 7539–7542. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Alix, A.; Kumarraja, M.; Louis, B.; Pale, P.; Sommer, J. Copper–Zeolites as Catalysts for the Coupling of Terminal Alkynes: An Efficient Synthesis of Diynes. Eur. J. Org. Chem. 2009, 2009, 423–429. [Google Scholar] [CrossRef]
- Kuhn, P.; Pale, P.; Sommer, J.; Louis, B. Probing Cu-USY Zeolite Reactivity: Design of a Green Catalyst for the Synthesis of Diynes. J. Phys. Chem. C 2009, 113, 2903–2910. [Google Scholar] [CrossRef]
- Chassaing, S.; Alix, A.; Boningari, T.; Sido, K.S.S.; Keller, M.; Kuhn, P.; Louis, B.; Sommer, J.; Pale, P. Copper(I)-Zeolites as New Heterogeneous and Green Catalysts for Organic Synthesis. Synthesis 2010, 2010, 1557–1567. [Google Scholar] [CrossRef]
- Heydari, N.; Bikas, R.; Siczek, M.; Lis, T. Green carbon–carbon homocoupling of terminal alkynes by a silica supported Cu(ii)-hydrazone coordination compound. Dalton Trans. 2023, 52, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, N.; Karthik, M.; Suresh, P. Copper catalyzed oxidative homocoupling of terminal alkynes to 1,3-diynes: A Cu3(BTC)2 MOF as an efficient and ligand free catalyst for Glaser–Hay coupling. Org. Biomol. Chem. 2017, 15, 9191–9199. [Google Scholar] [CrossRef]
- Györke, G.; Dancsó, A.; Volk, B.; Hunyadi, D.; Szalóki, I.; Milen, M. Copper-Containing Mineral Mediated Glaser Coupling of Terminal Alkynes. ChemistrySelect 2022, 7, e202200480. [Google Scholar] [CrossRef]
- Li, J.H.; Jiang, H.F. Glaser coupling reaction in supercritical carbon dioxide. Chem. Commun. 1999, 2369–2370. [Google Scholar] [CrossRef]
- Jiang, H.F.; Tang, J.Y.; Wang, A.Z.; Deng, G.H.; Yang, S.R. Cu(II)-Promoted Oxidative Homocoupling Reaction of Terminal Alkynes in Supercritical Carbon Dioxide. Synthesis 2006, 2006, 1155–1161. [Google Scholar] [CrossRef]
- Zhou, L.; Zhan, H.Y.; Liu, H.L.; Jiang, H.F. An Efficient and Practical Process for Pd/Cu CocatalyzedHomocoupling Reaction of Terminal Alkynes Using Sodium Percarbonate as a Dual Reagent in Aqueous Media. Chin. J. Chem. 2007, 25, 1413–1416. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Homocoupling reaction of terminal alkynes catalyzed by a reusable cationic 2,2′-bipyridyl palladium(II)/CuI system in water. Green Chem. 2009, 11, 269–274. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Reddy, B.K.; Uma, K.; Prasad, G.A.R. Glaser oxidative coupling in ionic liquids: An improved synthesis of conjugated 1,3-diynes. Tetrahedron Lett. 2003, 44, 6493–6496. [Google Scholar] [CrossRef]
- Ranu, B.C.; Banerjee, S. Homocoupling of terminal alkynes to 1,4-disubstituted 1,3-diynes promoted by copper(I) iodide and a task specific ionic liquid, [bmim]OH -A green procedure. Lett. Org. Chem. 2006, 3, 607–609. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Wang, L.; Pagni, R.M. Microwave Enhanced Glaser Coupling Under Solvent Free Conditions. Synlett 2001, 2001, 108–110. [Google Scholar] [CrossRef]
- Stolle, A.; Ondruschka, B. Solvent-free reactions of alkynes in ball mills: It is definitely more than mixing. Pure Appl. Chem. 2011, 83, 1343–1349. [Google Scholar] [CrossRef]
- Bag, S.S.; Sinha, S.; Singh, S.; Golder, A.K. Greener photocatalytic route to the hetero-selective Glaser coupling reaction: Role of hole/oxygen in air. Catal. Sci. Technol. 2023, 13, 1281–1287. [Google Scholar] [CrossRef]
- Yan, X.-M.; Chen, Z.-M.; Yang, F.; Huang, Z.-Z. A Dehydrogenative Homocoupling Reaction for the Direct Synthesis of Hydrazines from N-Alkylanilines in Air. Synlett 2011, 2011, 569–572. [Google Scholar] [CrossRef]
- Cicco, L.; Roggio, M.; López-Aguilar, M.; Ramos-Martín, M.; Perna, F.M.; García-Álvarez, J.; Vitale, P.; Capriati, V. Selective Aerobic Oxidation of Alcohols in Low Melting Mixtures and Water and Use for Telescoped One-Pot Hybrid Reactions. ChemistryOpen 2022, 11, e202200160. [Google Scholar] [CrossRef]
- Silva, E.D.; Alves, O.A.L.; Ribeiro, R.T.; Chagas, R.C.R.; Villar, J.A.F.P.; Princival, J.L. Homogeneous CuCl2/TMEDA/TEMPO-Catalyzed chemoselective base- and halogen- free aerobic oxidation of primary alcohols in mild conditions. Appl. Cat. A-Gen. 2021, 623, 118289. [Google Scholar] [CrossRef]
- Shastin, A.V.; Muzalevskii, V.M.; Korotchenko, V.N.; Balenkova, E.S.; Nenaidenko, V.G. Effect of the Catalyst Nature and Quantity on Catalytic Olefination. Russ. J. Org. Chem. 2006, 42, 183–189. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, S.; Luo, Q.; Zhang, J.; Guo, Q.; Zhang, Y.; Chai, X. Chemical and Pharmacological Progress on Polyacetylenes Isolated from the Family Apiaceae. Chem. Biodivers. 2015, 12, 474–502. [Google Scholar] [CrossRef]
- Ki, D.-W.; El-Desoky, A.H.; Wong, C.P.; Abdel-Ghani, M.; El-Beih, A.A.; Mizuguchi, M.; Morita, H. New Cytotoxic Polyacetylene Alcohols from the Egyptian Marine Sponge Siphonochalina Siphonella. J. Nat. Med. 2020, 74, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, C. Polyacetylenes in Herbal Medicine: A Comprehensive Review of Its Occurrence, Pharmacology, Toxicology, and Pharmacokinetics (2014–2021). Phytochemistry 2022, 201, 113288. [Google Scholar] [CrossRef] [PubMed]
- Seavill, P.W.; Holt, K.B.; Wilden, J.D. Investigations into the mechanism of copper-mediated Glaser–Hay couplings using electrochemical techniques. Faraday Discuss. 2019, 220, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Muzalevskiy, V.; Sizova, Z.; Shastin, A.; Nenajdenko, V.G.; Diusenov, A.I. Efficient multi gram approach to acetylenes and CF3-ynones starting from dichloroalkenes prepared by catalytic olefination reaction (COR). Eur. J. Org. Chem. 2020, 2020, 4161–4166. [Google Scholar] [CrossRef]
- Hosseini, A.; Seidel, D.; Miska, A.; Schreiner, P.R. Fluoride-Assisted Activation of Calcium Carbide: A Simple Method for the Ethynylation of Aldehydes and Ketones. Org. Lett. 2015, 17, 2808–2811. [Google Scholar] [CrossRef] [PubMed]
- Carbon Tetrachloride. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/carbon-tetrachloride.pdf (accessed on 20 June 2023 ).
- Rao, M.L.N.; Dasgupta, P.; Ramakrishna, B.S.; Murty, V.N. Domino Synthesis of 1,3-Diynes from 1,1-Dibromoalkenes: A Pd-Catalyzed Copper-Free Coupling Method. Tetrahedron Lett. 2014, 55, 3529–3533. [Google Scholar] [CrossRef]
- Feng, L.; Hu, T.; Zhang, S.; Xiong, H.-Y.; Zhang, G. Copper-Mediated Deacylative Coupling of Ynones via C–C Bond Activation under Mild Conditions. Org. Lett. 2019, 21, 9487–9492. [Google Scholar] [CrossRef]
- Shi, X.-L.; Hu, Q.; Wang, F.; Zhang, W.; Duan, P. Application of the Polyacrylonitrile Fiber as a Novel Support for Polymer-Supported Copper Catalysts in Terminal Alkyne Homocoupling Reactions. J. Catal. 2016, 337, 233–239. [Google Scholar] [CrossRef]
- Liu, D.-X.; Li, F.-L.; Li, H.-X.; Gao, J.; Lang, J.-P. Synthesis of 1,4-Diarylsubstituted 1,3-Diynes through Ligand-Free Copper-Catalyzed Oxidative Decarboxylative Homocoupling of Aryl Propiolic Acids. Tetrahedron 2014, 70, 2416–2421. [Google Scholar] [CrossRef]
- Stein, P.M.; Pascher, J.; Stracke, J.; Levacher, V.S.; Wagner, J.A.; Rominger, F.; Oeser, T.; Rudolph, M.; Hashmi, A.S.K. Gold Catalysis: 2,3- or 1,4-Addition to Butadiynes. Adv. Synth. Catal. 2022, 364, 3817–3839. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar Chattopadhyay, S. Transition-Metal-Free Synthesis of Symmetrical 1,4-Diarylsubstituted 1,3-Diynes by Iodine-Mediated Decarboxylative Homocoupling of Arylpropiolic Acids. Tetrahedron Lett. 2022, 102, 153908. [Google Scholar] [CrossRef]
- Sontakke, G.S.; Ghosh, C.; Pal, K.; Volla, C.M.R. Regioselective Dichotomy in Ru(II)-Catalyzed C–H Annulation of Aryl Pyrazolidinones with 1,3-Diynes. J. Org. Chem. 2022, 87, 14103–14114. [Google Scholar] [CrossRef]
- Jasiobedzki, W.; Moszczynski-Petkowski, R.; Wozniak-Kornacka, J. Diacetylene ω-glycols. Etherates—Adducts of 1,6-di(p-halophenyl)-1,6-diphenylhexa-2,4-diyne-1,6-diols with diethyl ether and dioxane. Bull. Pol. Acad. Sci. Chem. 2001, 49, 225–234. [Google Scholar]
- Ali, M.; Latif, A.; Bibi, S.; Ali, S.; Ali, A.; Ahmad, M.; Ahmad, R.; Khan, A.A.; Khan, A.; Ribeiro, A.I.; et al. Facile Synthesis of the Shape-Persistent 4-Hydroxybenzaldehyde Based Macrocycles and Exploration of their Key Electronic Properties: An Experimental and DFT Approach. ChemistrySelect 2022, 7, e202102715. [Google Scholar] [CrossRef]
- Ye, X.; Zhao, P.; Zhang, S.; Zhang, Y.; Wang, Q.; Shan, C.; Wojtas, L.; Guo, H.; Chen, H.; Shi, X. Facilitating Gold Redox Catalysis with Electrochemistry: An Efficient Chemical-Oxidant-Free Approach. Angew. Chem. Int. Ed. 2019, 58, 17226–17230. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzalevskiy, V.M.; Shastin, A.V.; Tirkasheva, S.I.; Ziyadullaev, O.E.; Parmanov, A.B.; Nenajdenko, V.G. CCl4-TMEDA-CuCl—A Novel Convenient Catalytic System for Dimerization of Terminal Acetylenes in Mild Conditions. Catalysts 2023, 13, 1330. https://doi.org/10.3390/catal13101330
Muzalevskiy VM, Shastin AV, Tirkasheva SI, Ziyadullaev OE, Parmanov AB, Nenajdenko VG. CCl4-TMEDA-CuCl—A Novel Convenient Catalytic System for Dimerization of Terminal Acetylenes in Mild Conditions. Catalysts. 2023; 13(10):1330. https://doi.org/10.3390/catal13101330
Chicago/Turabian StyleMuzalevskiy, Vasiliy M., Alexey V. Shastin, Sarvinoz I. Tirkasheva, Odiljon E. Ziyadullaev, Askar B. Parmanov, and Valentine G. Nenajdenko. 2023. "CCl4-TMEDA-CuCl—A Novel Convenient Catalytic System for Dimerization of Terminal Acetylenes in Mild Conditions" Catalysts 13, no. 10: 1330. https://doi.org/10.3390/catal13101330
APA StyleMuzalevskiy, V. M., Shastin, A. V., Tirkasheva, S. I., Ziyadullaev, O. E., Parmanov, A. B., & Nenajdenko, V. G. (2023). CCl4-TMEDA-CuCl—A Novel Convenient Catalytic System for Dimerization of Terminal Acetylenes in Mild Conditions. Catalysts, 13(10), 1330. https://doi.org/10.3390/catal13101330