Highly Efficient Catalytic Hydrodeoxygenation for Aliphatic Acid to Liquid Alkane: The Role of Molybdenum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Synthesis and Characterization
2.2. Evaluation of the Ni5Mox/SiO2 Catalysts
2.3. DFT Calculation
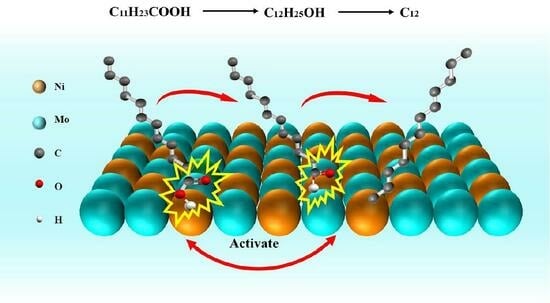
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Reactions and Product Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic hydrodeoxygenation of triglycerides: An approach to clean diesel fuel production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, D.; Chen, B. Metasilicate-based catalyst prepared from natural diatomaceous earth for biodiesel production. Renew. Energy 2019, 138, 1042–1050. [Google Scholar] [CrossRef]
- Zhao, N.; Zheng, Y.; Chen, J. Remarkably reducing carbon loss and H2 consumption on Ni–Ga intermetallic compounds in deoxygenation of methyl esters to hydrocarbons. J. Energy Chem. 2020, 41, 194–208. [Google Scholar] [CrossRef]
- Lang, M.; Li, H. Heterogeneous metal-based catalysts for cyclohexane synthesis from hydrodeoxygenation of lignin-derived phenolics. Fuel 2023, 344, 128084. [Google Scholar] [CrossRef]
- Kordouli, E.; Pawelec, B.; Fierro, J.L.G.; Lycourghiotis, A. Mo promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Appl. Catal. B Environ. 2018, 229, 139–154. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Z.; Yang, H.; Venderbosch, R.H.; Heeres, H.J. Catalytic Hydrotreatment of Biomass-Derived Fast Pyrolysis Liquids Using Ni and Cu-Based PRICAT Catalysts. Energy. Fuels 2022, 36, 14281–14291. [Google Scholar] [CrossRef]
- Di, L.; Yao, S.; Song, S. Robust ruthenium catalysts for the selective conversion of stearic acid to diesel-range alkanes. Appl. Catal. B Environ. 2017, 201, 137–149. [Google Scholar] [CrossRef]
- Chen, J.; Shi, H.; Li, L. Deoxygenation of methyl laurate as a model compound to hydrocarbons on transition metal phosphide catalysts. Appl. Catal. B Environ. 2014, 144, 870–884. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, S. Chemoselective hydrodeoxygenation of carboxylic acids to hydrocarbons over nitrogen-doped carbon-alumina hybrid supported iron catalysts. ACS. Catal. 2019, 9, 1564–1577. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Z.; Wu, K.; Shi, Y.; Pu, W.; Yang, M.; Wu, Y. Improved conversion of stearic acid to diesel-like hydrocarbons by carbon nanotubes-supported CuCo catalysts. Fuel Process. Technol. 2019, 188, 153–163. [Google Scholar] [CrossRef]
- Zhou, L.; Lawal, A. Hydrodeoxygenation of microalgae oil to green diesel over Pt, Rh and presulfided NiMo catalysts. Catal. Sci. Technol. 2016, 6, 1442–1454. [Google Scholar] [CrossRef]
- Nimkarde, M.R.; Vaidya, P.D. Toward Diesel Production from Karanja Oil Hydrotreating over CoMo and NiMo Catalysts. Energy Fuels 2016, 30, 3107–3112. [Google Scholar] [CrossRef]
- Yenumala, S.R.; Maity, S.K.; Shee, D. Reaction mechanism and kinetic modeling for the hydrodeoxygenation of triglycerides over alumina supported nickel catalyst. React. Kinet. Mech. Catal. 2017, 120, 109–128. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Zhang, M.; Wang, D.; Li, S.; Zhang, C. Preparation of reduced Ni-Nb-O composite hydrogenation catalysts for highly selective conversion of free fatty acids to n-alkanes. Fuel 2020, 282, 118842. [Google Scholar] [CrossRef]
- Rozmysłowicz, B.; Mäki-Arvela, P. Influence of Hydrogen in Catalytic Deoxygenation of Fatty Acids and Their Derivatives over Pd/C. Ind. Eng. Chem. 2012, 51, 8922–8927. [Google Scholar] [CrossRef]
- Zhou, L.; Lawal, A. Kinetic study of hydrodeoxygenation of palmitic acid as a model compound for microalgae oil over Pt/γ-Al2O3. Appl. Catal. A Gen. 2017, 532, 40–49. [Google Scholar] [CrossRef]
- Immer, J.G.; Kelly, M.J.; Lamb, H.H. Catalytic reaction pathways in liquid-phase deoxygenation of C18 free fatty acids. Appl. Catal. A Gen. 2010, 375, 134–139. [Google Scholar] [CrossRef]
- Grosso Giordano, N.A.; Eaton, T.R.; Bo, Z. Silica support modifications to enhance Pd-catalyzed deoxygenation of stearic acid. Appl. Catal. B Environ. 2016, 192, 93–100. [Google Scholar] [CrossRef]
- Ding, S.; Li, Z.; Li, F.; Wang, Z.; Li, J.; Zhao, T.; Lin, H. Catalytic hydrogenation of stearic acid over reduced NiMo catalysts: Structure–activity relationship and effect of the hydrogen-donor. Appl. Catal. A Gen. 2018, 566, 146–154. [Google Scholar] [CrossRef]
- Liu, S.; Simonetti, T.; Zheng, W.; Saha, B. Selective Hydrodeoxygenation of Vegetable Oils and Waste Cooking Oils to Green Diesel Using a Silica-Supported Ir-ReOx Bimetallic Catalyst. ChemSusChem 2018, 11, 1446–1454. [Google Scholar] [CrossRef]
- Kong, L.P.; Liu, C.Z.; Gao, J.; Wang, Y.Y.; Dai, L.Y. Efficient and controllable alcoholysis of Kraft lignin catalyzed by porous zeolite-supported nickel-copper catalyst. Bioresour. Technol. 2019, 276, 310–317. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, X.; Xie, F.; Jin, Z.; Song, X. Plasmonic Hybrid Mo/MoO2 Nanospheres as Surface-Enhanced Raman Scattering Substrates for Molecular Detection. ACS Appl. Nano Mater. 2020, 3, 5656–5664. [Google Scholar] [CrossRef]
- Leng, S.; Wang, X.; He, X.; Liu, L.; Liu, Y.; Zhuang, G.; Wang, G. NiFe/gamma -Al2O3:A universal catalyst for the hydrodeoxygenation of bio-oil and its model compounds. Catal. Commun. 2013, 41, 34–37. [Google Scholar] [CrossRef]
- Xia, H.; Li, J.; Chen, C.; Wu, D.; Ren, J.; Jiang, J. Selective aqueous-phase hydrogenation of furfural to cyclopentanol over Ni-based catalysts prepared from Ni-MOF composite. Inorg. Chem. Commun. 2021, 133, 108894. [Google Scholar] [CrossRef]
- Han, D.P.; Yin, W.; Luo, D.; He, H.; Wang, S.P.; Xia, S.Q. Hydrodeoxygenation of aliphatic acid over NiFe intermetallic compounds: Insights into the mechanism via model compound study. Fuel 2021, 305, 121545. [Google Scholar] [CrossRef]
- Chakthranont, P.; Sattayanon, C.; Butburee, T.; Faungnawakij, K. Understanding the promoter effect of bifunctional (Pt, Ni, Cu)-MoO3-x/TiO2 catalysts for the hydrodeoxygenation of p-cresol: A combined DFT and experimental study. Appl. Surf. Sci. 2021, 547, 149170. [Google Scholar]
- Bergstrom, O.; Andersson, A.M.; Edstromm, K.; Gustafsson, T.J. A neutron diffraction cell for studying lithium-insertion processes in electrode materials. Appl. Crystallogr. 1998, 31, 823–825. [Google Scholar] [CrossRef]
- Han, D.; Yin, W.; Arslan, A.; Liu, T.; Zheng, Y.; Xia, S. Stabilization of Fast Pyrolysis Liquids from Biomass by Mild Catalytic Hydrotreatment: Model Compound Study. Catalysts 2020, 10, 402. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, G.; Liu, X.; Yu, X.; Liu, L.J. Structure and Reducibility of NiO-MoO3/γ-Al2O3 Catalysts: Effects of Loading and Molar Ratio. Phys. Chem. C. 2008, 112, 17265–17271. [Google Scholar] [CrossRef]
- Ni, J.; Wang, G.; Yang, J.; Gao, D.; Chen, J. Carbon nanotube-wired and oxygen-deficient MoO3 nanobelts with enhanced lithium-storage capability. J Power Sources 2014, 247, 90–94. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Zeng, H.; Zhao, H.; Sun, W. Hierarchical Porous NiO/beta-NiMoO4 Heterostructure as Superior Anode Material for Lithium Storage. ChemPlusChem. 2018, 83, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Tyrone Ghampson, I.; Yun, G.; Hwang, D. A significant support effect on RuSn catalysts for carboxylic acid transformation to hydrocarbons. Chem. Eng. J. 2023, 461, 141912. [Google Scholar]
- Lu, Y.; Guo, D.; Zhao, Y.; Wang, S.; Ma, X. Enhanced performance of xNi@yMo-HSS catalysts for DRM reaction via the formation of a novel SiMoOx species. Appl. Catal. B. 2021, 291, 120075. [Google Scholar] [CrossRef]
- Petropoulos, G.; Bourikas, K. Transformation of vegetable oils into green diesel over Ni-Mo catalysts supported on titania. Catal. Today 2023, 423, 114268. [Google Scholar] [CrossRef]
- Nikolopoulos, N.; Kordulis, C. Mo promoted Ni-ZrO2 co-precipitated catalysts for green diesel production. Chem. Eng. Sci. 2023, 270, 118540. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Kharkar, M.; Yadav, G.; Rajadhyaksha, R.A. Geometric and electronic effects in silica supported bimetallic nickel–copper and nickel–iron catalysts for liquid-phase hydrogenation of acetophenone and benzonitrile. J. Chem. Soc. Chem. Commun. 1992, 7, 584–586. [Google Scholar] [CrossRef]
- Cao, X.; Long, F.; Zhai, Q.; Zhao, J.; Jiang, J. Heterogeneous Ni and MoOx co-loaded CeO2 catalyst for the hydrogenation of fatty acids to fatty alcohols under mild reaction conditions. Fuel 2021, 298, 120829. [Google Scholar] [CrossRef]
- Kumar, P.; Maity, S.K.; Shee, D. Role of NiMo Alloy and Ni Species in the Performance of NiMo/Alumina Catalysts for Hydrodeoxygenation of Stearic Acid: A Kinetic Study. ACS Omega 2019, 4, 2833–2843. [Google Scholar] [CrossRef]
- Olorunyolemi, T.; Kydd, R.A. Laser Raman spectroscopy of MoO3 and NiO-MoO3 supported on gallia and gallium-aluminum mixed oxides. Catal. Lett. 2000, 65, 185–192. [Google Scholar] [CrossRef]
- Maione, A.; Devillers, M.J. Solid solutions of Ni and Co molybdates in silica-dispersed and bulk catalysts prepared by sol–gel and citrate methods. Solid State Chem. 2004, 177, 2339–2349. [Google Scholar] [CrossRef]
- Abdel-Dayem, H.M. Dynamic phenomena during reduction of alpha-NiMoO4 in different atmospheres: In-situ thermo-Raman spectroscopy study. Ind. Eng. Chem. Res. 2007, 46, 2466–2472. [Google Scholar] [CrossRef]
- Araujo, R.B.; Rodrigues, G.L.S. Adsorption energies on transition metal surfaces: Towards an accurate and balanced description. Nat. Commun. 2022, 13, 6853. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Ge, Q.J. Methanol Adsorption on the Clean CeO2(111) Surface: A Density Functional Theory Study. Phys. Chem. C. 2007, 111, 10514–10522. [Google Scholar] [CrossRef]
- Loe, R.; Morgan, T.; Sewell, L.; Ji, Y.Y.; Lee, A.F. Effect of Cu and Sn promotion on the catalytic deoxygenation of model and algal lipids to fuel-like hydrocarbons over supported Ni catalysts. Appl. Catal. B Environ. 2016, 191, 147–156. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Kharel, P.R.; Boakye, E. Hydrocarbon bio-oil production from pyrolysis bio-oil using non-sulfide Ni-Zn/Al2O3 catalyst. Fuel Process. Technol. 2017, 162, 78–86. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 1758. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B. 1993, 48, 13115–13118. [Google Scholar] [CrossRef] [PubMed]














| Theory Metal Loading, wt% | Actual Metal Loading, wt% 1 | Dispersity, % 2 | |||
|---|---|---|---|---|---|
| Ni | Mo | Ni | Mo | ||
| Ni | 10 | - | 10.55 | - | 35.40 |
| Ni5Mo1 | 10 | 2 | 9.88 | 1.45 | 36.05 |
| Ni5Mo3 | 10 | 6 | 10.65 | 5.20 | 17.89 |
| Ni5Mo5 | 10 | 10 | 10.47 | 8.70 | 17.85 |
| Ni5Mo7 | 10 | 14 | 9.28 | 10.24 | 10.25 |
| Ni5Mo10 | 10 | 20 | 9.66 | 16.23 | 9.80 |
| Mo | - | 10 | - | 10.12 | 0.19 |
| Catalysts | Ni0 | Ni2+ | Mo0 | Mo3+ | Mo4+ | Mo5+ | Mo6+ |
|---|---|---|---|---|---|---|---|
| Ni | 9.55 | 90.45 | - | - | - | - | - |
| Ni5Mo1 | 19.44 | 80.56 | 0.00 | 16.81 | 17.92 | 24.85 | 40.42 |
| Ni5Mo3 | 16.61 | 83.39 | 4.72 | 10.57 | 27.62 | 17.42 | 39.67 |
| Ni5Mo5 | 17.53 | 82.47 | 5.52 | 16.32 | 25.49 | 19.89 | 32.79 |
| Ni5Mo7 | 12.57 | 87.43 | 8.15 | 16.15 | 25.10 | 25.58 | 25.02 |
| Mo | - | - | 7.54 | 19.91 | 28.21 | 25.72 | 18.62 |
| Ni (111) | MoO2 (011) | Ni5/MoO2 | ||
|---|---|---|---|---|
| MoO2 Site | Ni Site | |||
| Methanol | −0.66 eV | −1.81eV | −1.61 eV | −0.88 eV |
| H | −0.64 eV | 0.10 eV | 0.13 eV | −0.26 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Han, D.; Xia, S. Highly Efficient Catalytic Hydrodeoxygenation for Aliphatic Acid to Liquid Alkane: The Role of Molybdenum. Catalysts 2023, 13, 1329. https://doi.org/10.3390/catal13101329
Li J, Han D, Xia S. Highly Efficient Catalytic Hydrodeoxygenation for Aliphatic Acid to Liquid Alkane: The Role of Molybdenum. Catalysts. 2023; 13(10):1329. https://doi.org/10.3390/catal13101329
Chicago/Turabian StyleLi, Jiangtao, Depeng Han, and Shuqian Xia. 2023. "Highly Efficient Catalytic Hydrodeoxygenation for Aliphatic Acid to Liquid Alkane: The Role of Molybdenum" Catalysts 13, no. 10: 1329. https://doi.org/10.3390/catal13101329
APA StyleLi, J., Han, D., & Xia, S. (2023). Highly Efficient Catalytic Hydrodeoxygenation for Aliphatic Acid to Liquid Alkane: The Role of Molybdenum. Catalysts, 13(10), 1329. https://doi.org/10.3390/catal13101329