ZnO-Bi2O3 Heterostructured Composite for the Photocatalytic Degradation of Orange 16 Reactive Dye: Synergistic Effect of UV Irradiation and Hydrogen Peroxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Characterization of the ZnO-Bi2O3 Heterojunction
2.1.1. Crystalline Structure Using XRD Analysis
2.1.2. SEM Analysis
2.1.3. FTIR Spectroscopy
2.2. Evaluation of the Photocatalytic Activity of ZnO-Bi2O3
2.2.1. Effect of Sole UV Irradiation on the Photolytic Degradation of RO16 Dye
2.2.2. Effect of H2O2 Addition on the Degradation of RO16 under UV Irradiation
2.2.3. Evaluating the Photocatalytic Activity of ZnO, Bi2O3, and ZnO-Bi2O3
2.2.4. Effect of RO16 Dye Concentration on the Activity of the ZnO-Bi2O3 Photocatalyst under UV Irradiation
2.2.5. Effect of H2O2 on the Degradation Rate under UV Irradiation at ZnO-Bi2O3
2.2.6. Comparison of the Degradation Performance and Kinetics for RO16 under Different Reaction Conditions
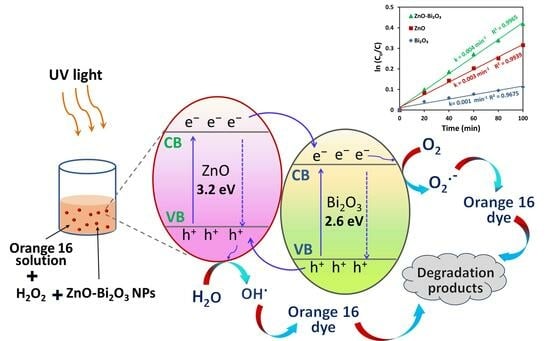
2.2.7. Proposed Mechanism of Photocatalytic Activity at ZnO-Bi2O3
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of ZnO-Bi2O3 Particles
3.3. Characterization of ZnO-Bi2O3 Particles
3.4. Photocatalytic Degradation Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhatia, S.; Devraj, S. Pollution Control in Textile Industry; WPI Publishing: New Delhi, India, 2017. [Google Scholar]
- Gita, S.; Hussan, A.; Choudhury, T. Impact of textile dyes waste on aquatic environments and its treatment. Environ. Ecol. 2017, 35, 2349–2353. [Google Scholar]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Amin, H.M.; El-Kady, M.F.; Atta, N.F.; Galal, A. Gold nanoparticles decorated graphene as a high performance sensor for determination of trace hydrazine levels in water. Electroanalysis 2018, 30, 1757–1766. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Slokar, Y.M.; Le Marechal, A.M. Methods of decoloration of textile wastewaters. Dyes Pigm. 1998, 37, 335–356. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Brüninghoff, R.; Van Duijne, A.K.; Braakhuis, L.; Saha, P.; Jeremiasse, A.W.; Mei, B.; Mul, G. Comparative analysis of photocatalytic and electrochemical degradation of 4-ethylphenol in saline conditions. Environ. Sci. Technol. 2019, 53, 8725–8735. [Google Scholar] [CrossRef]
- Premaratne, M.; Nishshanka, G.; Liyanaarachchi, V.; Nimarshana, P.; Ariyadasa, T.U. Bioremediation of textile dye wastewater using microalgae: Current trends and future perspectives. J. Chem. Technol. Biotechnol. 2021, 96, 3249–3258. [Google Scholar] [CrossRef]
- Dihom, H.R.; Al-Shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Khamidun, M.H.B. Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. J. Water Process. Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Zhang, Z.; Sun, W.; Xu, R.; Xie, Y.; Xing, Z.; Zhang, X. Efficient photocatalytic degradation of organic dyes over titanium dioxide coating upconversion luminescence agent under visible and sunlight irradiation. Appl. Catal. A Gen. 2008, 334, 227–233. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Amin, H.M. Synthesis and photoelectrochemical behavior of a hybrid electrode composed of polyaniline encapsulated in highly ordered TiO2 nanotubes array. Int. J. Electrochem. Sci. 2012, 7, 3610–3626. [Google Scholar]
- Atta, N.F.; Amin, H.M.; Khalil, M.W.; Galal, A. Nanotube arrays as photoanodes for dye sensitized solar cells using metal phthalocyanine dyes. Int. J. Electrochem. Sci. 2011, 6, 3. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.T.; Chen, J.F.; Tao, X. Enhanced photosensitized degradation of organic pollutants under visible radiation by (I2) n-encapsulated TiO2 films. Ind. Eng. Chem. Res. 2012, 51, 1110–1117. [Google Scholar] [CrossRef]
- Ghaffar, S.; Abbas, A.; Naeem-ul-Hassan, M.; Assad, N.; Sher, M.; Ullah, S.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Al Bratty, M.; et al. Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles. Antioxidants 2023, 12, 1201. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Patil, M.B.; Mathad, S.N.; Patil, A.Y.; Otaibi, A.A.; Masood, N.; Mansour, D.; Khan, A.; Manikandan, A.; Syafri, E. Photocatalytic Degradation of Textile Orange 16 Reactive Dye by ZnO Nanoparticles Synthesized via Green Route Using Punica Granatum Leaf Extract. Crystals 2023, 13, 172. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.-Z.; Kang, Y.-R.; Sun, Z.-S.; Wang, X.-D.; Meng, Y.; Hong, M.-H.; Xie, W.-F. Cauliflower-shaped Bi2O3–ZnO heterojunction with superior sensing performance towards ethanol. J. Alloys Compd. 2021, 854, 157152. [Google Scholar] [CrossRef]
- Toledo Arana, J.; Torres, J.J.; Acevedo, D.F.; Illanes, C.O.; Ochoa, N.A.; Pagliero, C.L. One-step synthesis of CaO-ZnO efficient catalyst for biodiesel production. Int. J. Chem. Eng. 2019, 2019, 1806017. [Google Scholar] [CrossRef]
- Balachandran, S.; Swaminathan, M. Facile fabrication of heterostructured Bi2O3–ZnO photocatalyst and its enhanced photocatalytic activity. J. Phys. Chem. C 2012, 116, 26306–26312. [Google Scholar] [CrossRef]
- Medina, J.; Portillo-Vélez, N.; Bizarro, M.; Hernández-Gordillo, A.; Rodil, S. Synergistic effect of supported ZnO/Bi2O3 heterojunctions for photocatalysis under visible light. Dyes Pigm. 2018, 153, 106–116. [Google Scholar] [CrossRef]
- Wen, L.; Wang, D.; Xi, J.; Tian, F.; Liu, P.; Bai, Z.-W. Heterometal modified Fe3O4 hollow nanospheres as efficient catalysts for organic transformations. J. Catal. 2022, 413, 779–785. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Wen, L.; Xi, J.; Liu, P.; Hansen, T.W.; Li, P. Ni-Pd-incorporated Fe3O4 yolk-shelled nanospheres as efficient magnetically recyclable catalysts for reduction of n-containing unsaturated compounds. Catalysts 2023, 13, 190. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Gou, L.; Wei, S.; Hou, X.; Wu, L. Au Nanoparticles Decorated CoP Nanowire Array: A Highly Sensitive, Anticorrosive, and Recyclable Surface-Enhanced Raman Scattering Substrate. Anal. Chem. 2023, 95, 11037–11046. [Google Scholar] [CrossRef] [PubMed]
- Mottola, S.; Mancuso, A.; Sacco, O.; Vaiano, V.; De Marco, I. Photocatalytic Systems Based on ZnO Produced by Supercritical Antisolvent for Ceftriaxone Degradation. Catalysts 2023, 13, 1173. [Google Scholar] [CrossRef]
- Boudalia, M.; Laourayed, M.; El Moudane, M.; Sekkat, Z.; Campos, O.S.; Bellaouchou, A.; Guenbour, A.; Garcia, A.J.; Amin, H.M. Phosphate glass doped with niobium and bismuth oxides as an eco-friendly corrosion protection matrix of iron steel in HCl medium: Experimental and theoretical insights. J. Alloys Compd. 2023, 938, 168570. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Liu, J.; Cheng, K.; Sun, W.; Lin, J. Enhanced visible light photocatalysis of Bi2O3 upon fluorination. J. Phys. Chem. C 2013, 117, 20029–20036. [Google Scholar] [CrossRef]
- Wang, X.; Ren, P.; Fan, H. Room-temperature solid state synthesis of ZnO/Bi2O3 heterojunction and their solar light photocatalytic performance. Mater. Res. Bull. 2015, 64, 82–87. [Google Scholar] [CrossRef]
- Amin, H.M.; Molls, C.; Bawol, P.P.; Baltruschat, H. The impact of solvent properties on the performance of oxygen reduction and evolution in mixed tetraglyme-dimethyl sulfoxide electrolytes for Li-O2 batteries: Mechanism and stability. Electrochim. Acta 2017, 245, 967–980. [Google Scholar] [CrossRef]
- Ramachandran, S.; Sivasamy, A. Effective charge separation in binary ZnO-Bi2O3 photocatalytic material for the treatment of simulated wastewater. Mater. Today Proc. 2019, 17, 101–110. [Google Scholar] [CrossRef]
- Georgiou, D.; Melidis, P.; Aivasidis, A.; Gimouhopoulos, K. Degradation of azo-reactive dyes by ultraviolet radiation in the presence of hydrogen peroxide. Dyes Pigm. 2002, 52, 69–78. [Google Scholar] [CrossRef]
- Ali, A.; Biswas, M.R.U.D.; Oh, W.C. Novel and simple process for the photocatalytic reduction of CO2 with ternary Bi2O3–graphene–ZnO nanocomposite. J. Mater. Sci. Mater. Electron. 2018, 29, 10222–10233. [Google Scholar] [CrossRef]
- Reddy, K.H.; Martha, S.; Parida, K. Facile fabrication of Bi2O3/Bi–NaTaO3 photocatalysts for hydrogen generation under visible light irradiation. RSC Adv. 2012, 2, 9423–9436. [Google Scholar] [CrossRef]
- Premalatha, N.; Miranda, L.R. Surfactant modified ZnO–Bi2O3 nanocomposite for degradation of lambda-cyhalothrin pesticide in visible light: A study of reaction kinetics and intermediates. J. Environ. Manag. 2019, 246, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, P.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P.; Rahmani-Sani, A.; Thakur, V.; Saini, A.K. Synthesis of eu3+− doped zno/bi2o3 heterojunction photocatalyst on graphene oxide sheets for visible light-assisted degradation of 2, 4-dimethyl phenol and bacteria killing. Solid State Sci. 2020, 102, 106164. [Google Scholar] [CrossRef]
- Saritha, D.; Markandeya, Y.; Salagram, M.; Vithal, M.; Singh, A.; Bhikshamaiah, G. Effect of Bi2O3 on physical, optical and structural studies of ZnO–Bi2O3–B2O3 glasses. J. Non Cryst. Solids 2008, 354, 5573–5579. [Google Scholar] [CrossRef]
- Ullah, S.; Khalid, R.; Rehman, M.F.; Irfan, M.I.; Abbas, A.; Alhoshani, A.; Anwar, F.; Amin, H. Biosynthesis of phyto-functionalized silver nanoparticles using olive fruit extract and evaluation of their antibacterial and antioxidant properties. Front. Chem. 2023, 11, 1202252. [Google Scholar] [CrossRef]
- Dhahri, I.; Ellouze, M.; Labidi, S.; Al-Bataineh, Q.M.; Etzkorn, J.; Guermazi, H.; Telfah, A.; Tavares, C.J.; Hergenröder, R.; Appel, T. Optical and structural properties of ZnO NPs and ZnO–Bi2O3 nanocomposites. Ceram. Int. 2022, 48, 266–277. [Google Scholar] [CrossRef]
- Pankove, J.I. Optical Processes in Semiconductors; Courier Corporation: Mineola, NY, USA, 1975. [Google Scholar]
- Jamal, M.A.; Muneer, M.; Iqbal, M. Photo-degradation of monoazo dye blue 13 using advanced oxidation process. Chem. Int 2015, 1, 12–16. [Google Scholar]
- Mitrović, J.Z.; Radović, M.D.; Anđelković, T.D.; Bojić, D.V.; Bojić, A.L. Identification of intermediates and ecotoxicity assessment during the UV/H2O2 oxidation of azo dye Reactive Orange 16. J. Environ. Sci. Health A 2014, 49, 491–502. [Google Scholar] [CrossRef]
- Bokhari, T.H.; Kashif, M.; Bhatti, I.A.; Zubair, M.; Adeel, S.; Yousaf, M.; Ahmad, M.; Iqbal, M.; Usman, M.; Zuber, M. Degradation Study of CI Reactive Yellow 145 by Advanced Oxidation Process. Asian J. Chem. 2013, 25, 8668–8672. [Google Scholar] [CrossRef]
- Medina, J.; Bizarro, M.; Gomez, C.; Depablos-Rivera, O.; Mirabal-Rojas, R.; Monroy, B.; Fonseca-Garcia, A.; Perez-Alvarez, J.; Rodil, S. Sputtered bismuth oxide thin films as a potential photocatalytic material. Catal. Today 2016, 266, 144–152. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO: Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L.; Lv, T.; Sun, Z.; Sun, C.Q. Visible light photocatalytic degradation of dyes by bismuth oxide-reduced graphene oxide composites prepared via microwave-assisted method. J. Colloid Interface Sci. 2013, 408, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Landge, V.; Sonawane, S.; Sivakumar, M.; Sonawane, S.; Babu, G.U.B.; Boczkaj, G. S-scheme heterojunction Bi2O3-ZnO/Bentonite clay composite with enhanced photocatalytic performance. Sustain. Energy Technol. Assess 2021, 45, 101194. [Google Scholar] [CrossRef]
- Foote, C.S.; Valentine, J.S.; Greenberg, A.; Liebman, J.F. Active Oxygen in Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 2. [Google Scholar]
- Guo, J.-G.; Liu, Y.; Hao, Y.-J.; Li, Y.-L.; Wang, X.-J.; Liu, R.-H.; Li, F.-T. Comparison of importance between separation efficiency and valence band position: The case of heterostructured Bi3O4Br/α-Bi2O3 photocatalysts. Appl. Catal. B Environ. 2018, 224, 841–853. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. J. Chem. Eng. 2022, 430, 132806. [Google Scholar] [CrossRef]
- Jan, T.; Azmat, S.; Wahid, B.; Adil, M.; Alawadhi, H.; Mansoor, Q.; Farooq, Z.; Ilyas, S.; Ahmad, I.; Ismail, M. Chemically synthesized ZnO-Bi2O3 (BZO) nanocomposites with tunable optical, photoluminescence and antibacterial characteristics. Mater. Sci. Semicond. Process. 2018, 84, 71–75. [Google Scholar] [CrossRef]











| UV without H₂O₂ | UV with H₂O₂ | ZnO-Bi₂O₃ +UV without H₂O₂ | ZnO-Bi₂O₃ + UV with H₂O₂ | |
|---|---|---|---|---|
| k (min−1) | 0.002 | 0.003 | 0.004 | 0.008 |
| R2 | 0.989 | 0.999 | 0.997 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzad, R.; Muneer, M.; Khalid, R.; Amin, H.M.A. ZnO-Bi2O3 Heterostructured Composite for the Photocatalytic Degradation of Orange 16 Reactive Dye: Synergistic Effect of UV Irradiation and Hydrogen Peroxide. Catalysts 2023, 13, 1328. https://doi.org/10.3390/catal13101328
Shahzad R, Muneer M, Khalid R, Amin HMA. ZnO-Bi2O3 Heterostructured Composite for the Photocatalytic Degradation of Orange 16 Reactive Dye: Synergistic Effect of UV Irradiation and Hydrogen Peroxide. Catalysts. 2023; 13(10):1328. https://doi.org/10.3390/catal13101328
Chicago/Turabian StyleShahzad, Roeel, Majid Muneer, Rimsha Khalid, and Hatem M. A. Amin. 2023. "ZnO-Bi2O3 Heterostructured Composite for the Photocatalytic Degradation of Orange 16 Reactive Dye: Synergistic Effect of UV Irradiation and Hydrogen Peroxide" Catalysts 13, no. 10: 1328. https://doi.org/10.3390/catal13101328
APA StyleShahzad, R., Muneer, M., Khalid, R., & Amin, H. M. A. (2023). ZnO-Bi2O3 Heterostructured Composite for the Photocatalytic Degradation of Orange 16 Reactive Dye: Synergistic Effect of UV Irradiation and Hydrogen Peroxide. Catalysts, 13(10), 1328. https://doi.org/10.3390/catal13101328