Ni-Doped La0.6Sr0.4CoO3 Perovskite as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions in Alkaline Media
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experiments
4.1. Preparation of the Perovskite Catalyst
4.2. Characterization of Perovskite Catalysts
4.3. Electrochemical Measurements
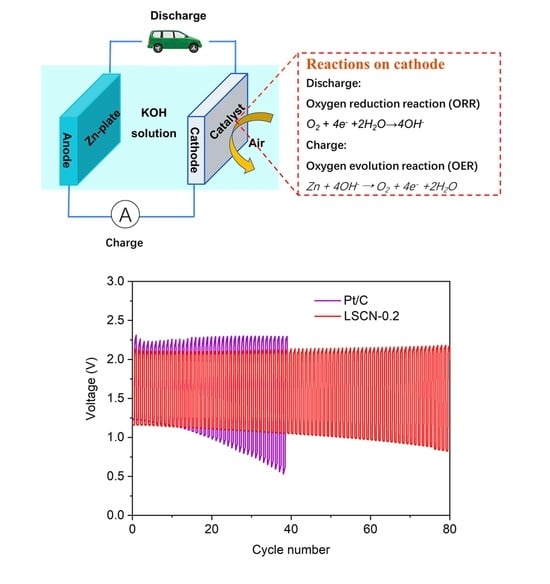
4.4. Preparation and Tests of the Zn-Air Battery
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, J.-S.; Kim, S.T.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Li, P.; Wei, B.; Lü, Z.; Wu, Y.; Zhang, Y.; Huang, X. La1.7Sr0.3Co0.5Ni0.5O4+δ layered perovskite as an efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Appl. Surf. Sci. 2019, 464, 494–501. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Dong, Y.; Su, Y.; Wei, C.; Chen, T.; Yan, W.; Ma, S.; Ma, L.; Wang, B.; Chen, L.; et al. Benchmarking the Effect of Particle Size on Silicon Anode Materials for Lithium-Ion Batteries. Small 2023, e2301301. [Google Scholar] [CrossRef] [PubMed]
- Asmontas, S.; Mujahid, M. Recent Progress in Perovskite Tandem Solar Cells. Nanomaterials 2023, 13, 1886. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wang, Z.; Wang, Q.; Sun, S.; Xue, Y.; Wang, F.; Zhao, J.; Liu, Z.; Yuan, J. A new family of Mn-based perovskite (La1−xYxMnO3) with improved oxygen electrocatalytic activity for metal-air batteries. Energy 2018, 154, 561–570. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Tan, P.; Chen, B.; Xu, H.; Zhang, H.; Cai, W.; Ni, M.; Liu, M.; Shao, Z. Flexible Zn– and Li–air batteries: Recent advances, challenges, and future perspectives. Energy Environ. Sci. 2017, 10, 2056–2080. [Google Scholar] [CrossRef]
- Fu, C.; Ma, Q.; Wu, Q.; Yuan, Z.; Wu, Z.; He, J.; Li, X. Boosting bifunctional catalytic activity of perovskite nanoparticles for rechargeable Zn-air batteries. Mater. Chem. Phys. 2022, 290, 126557. [Google Scholar] [CrossRef]
- Jung, K.N.; Jung, J.H.; Im, W.B.; Yoon, S.; Shin, K.H.; Lee, J.W. Doped lanthanum nickelates with a layered perovskite structure as bifunctional cathode catalysts for rechargeable metal-air batteries. ACS Appl. Mater. Interfaces 2013, 5, 9902–9907. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, H.; Sun, S.; Wang, Q.; Li, S.; Liu, Z. (La1−xSrx)0.98MnO3 perovskite with A-site deficiencies toward oxygen reduction reaction in aluminum-air batteries. J. Power Sources 2017, 342, 192–201. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef]
- Zamani-Meymian, M.R.; Chenab, K.K.; Pourzolfaghar, H. Designing High-Quality Electrocatalysts Based on CoO:MnO2@C Supported on Carbon Cloth Fibers as Bifunctional Air Cathodes for Application in Rechargeable Zn-Air Battery. ACS Appl. Mater. Interfaces 2022, 14, 55594–55607. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Wang, L.; Fu, H. Structural Design Strategy and Active Site Regulation of High-Efficient Bifunctional Oxygen Reaction Electrocatalysts for Zn-Air Battery. Small 2021, 17, e2006766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Cheng, Y.; Dou, S.; Veder, J.P.; Wang, S.; Saunders, M.; Jiang, S.P. Efficient and Durable Bifunctional Oxygen Catalysts Based on NiFeO@MnOx Core-Shell Structures for Rechargeable Zn-Air Batteries. ACS Appl. Mater. Interfaces 2017, 9, 8121–8133. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cheng, F.; Zhang, T.; Yang, J.; Hu, Y.; Chen, J. Hydrogenated uniform Pt clusters supported on porous CaMnO3 as a bifunctional electrocatalyst for enhanced oxygen reduction and evolution. Adv. Mater. 2014, 26, 2047–2051. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.B. Recent Advances in Noble Metal (Pt, Ru, and Ir)-Based Electrocatalysts for Efficient Hydrogen Evolution Reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Goh, K.; Zhao, L.; Sui, X.L.; Gong, X.F.; Cai, J.J.; Zhou, Q.Y.; Zhang, H.D.; Li, L.; Kong, F.R.; et al. Advanced non-noble materials in bifunctional catalysts for ORR and OER toward aqueous metal-air batteries. Nanoscale 2020, 12, 21534–21559. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, Z.; Ge, X.; Yang, S.; Peng, Y.; Kang, Z.; Liu, Z.; Lee, J.Y.; Zhao, D. A metal-free ORR/OER bifunctional electrocatalyst derived from metal-organic frameworks for rechargeable Zn-Air batteries. Carbon 2017, 111, 641–650. [Google Scholar] [CrossRef]
- Dai, L.; Liu, M.; Song, Y.; Liu, J.; Wang, F. Mn3O4-decorated Co3O4 nanoparticles supported on graphene oxide: Dual electrocatalyst system for oxygen reduction reaction in alkaline medium. Nano Energy 2016, 27, 185–195. [Google Scholar] [CrossRef]
- Ishihara, T.; Guo, L.M.; Miyano, T.; Inoishi, Y.; Kaneko, K.; Ida, S. Mesoporous La0.6Ca0.4CoO3 perovskites with large surface areas as stable air electrodes for rechargeable Zn–air batteries. J. Mater. Chem. A 2018, 6, 7686–7692. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Han, Z.; Yang, Y.; Li, J.; Deng, Q. Recent Advances in Oxygen Electrocatalysts Based on Perovskite Oxides. Nanomaterials 2019, 9, 1161. [Google Scholar] [CrossRef]
- Risch, M.; Stoerzinger, K.A.; Maruyama, S.; Hong, W.T.; Takeuchi, I.; Shao-Horn, Y. La0.8Sr0.2MnO3-delta decorated with Ba0.5Sr0.5Co0.8Fe0.2O3-delta: A bifunctional surface for oxygen electrocatalysis with enhanced stability and activity. J. Am. Chem. Soc. 2014, 136, 5229–5232. [Google Scholar] [CrossRef]
- Kolla, P.; Nasymov, G.; Rajappagowda, R.; Smirnova, A. Bi-functionality of samarium- and praseodymium-based perovskite catalysts for oxygen reduction and oxygen evolution reactions in alkaline medium. J. Power Sources 2020, 446, 227234. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Yu, L.; Zhang, J.; Feng, J.; Zhao, L. Engineering of the A-site deficiency in La0.4Sr0.6Co0.7Fe0.2Nb0.1O3-δ perovskites for enhanced elelctrocatalytic oxygen reduction reaction. J. Electroanal. Chem. 2022, 926, 116936. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, H.; Qian, B.; Wang, S.; Zheng, Y.; Ge, L.; Chen, H. Y and Fe co-doped LaNiO3 perovskite as a novel bifunctional electrocatalyst for rechargeable zinc-air batteries. Int. J. Hydrogen Energy 2023, 48, 8082–8092. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, A.; Yu, Y.; Tan, R.; Liu, C.; Zhang, P.; Liu, D.; Gui, J. Engineering Oxygen Vacancies into LaCoO3 Perovskite for Efficient Electrocatalytic Oxygen Evolution. ACS Sustain. Chem. Eng. 2018, 7, 2906–2910. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Wang, Q.; Wang, X.; Qian, D.; Jiang, J.; Li, J.; Chen, Z. Designed synthesis of LaCoO3/N-doped reduced graphene oxide nanohybrid as an efficient bifunctional electrocatalyst for ORR and OER in alkaline medium. J. Alloys Compd. 2017, 725, 260–269. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, Y.; Zhang, Z.; Li, J.; Qian, J.; Gao, D. Cation substitution of B-site in LaCoO3 for bifunctional oxygen electrocatalytic activities. J. Alloys Compd. 2021, 878, 160433. [Google Scholar] [CrossRef]
- Chandrappa, S.G.; Moni, P.; Chen, D.; Karkera, G.; Prakasha, K.R.; Caruso, R.A.; Prakash, A.S. The influence of ruthenium substitution in LaCoO3 towards bi-functional electrocatalytic activity for rechargeable Zn–air batteries. J. Mater. Chem. A 2020, 8, 20612–20620. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Xia, B.; Zhang, J.; Zhang, Z.; Guan, C.; Gao, D.; Huang, W. Multi-stability modulating of alkaline-earth metal doped LaCoO3 for rechargeable Zn-air batteries. Energy Storage Mater. 2021, 42, 470–476. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, W.; Lu, Z.; He, S.; Yin, B.; Zheng, Y.; Ge, L.; Chen, H. Ba-doped LaCoO3 perovskite as novel bifunctional electrocatalyst for zinc–air batteries. Electrochim. Acta 2023, 462, 142757. [Google Scholar] [CrossRef]
- Shin, B.; Choi, S. Electrocatalytic Activity of Co-based Perovskite Oxides for Oxygen Reduction and Evolution Reactions. Int. J. Electrochem. Sci. 2016, 11, 5900–5908. [Google Scholar] [CrossRef]
- Ananyev, M.V.; Porotnikova, N.M.; Kurumchin, E.K. Influence of strontium content on the oxygen surface exchange kinetics and oxygen diffusion in La1–xSrxCoO3–δ oxides. Solid State Ion. 2019, 341, 115052. [Google Scholar] [CrossRef]
- Wang, M.; Han, B.; Deng, J.; Jiang, Y.; Zhou, M.; Lucero, M.; Wang, Y.; Chen, Y.; Yang, Z.; N’Diaye, A.T.; et al. Influence of Fe Substitution into LaCoO3 Electrocatalysts on Oxygen-Reduction Activity. ACS Appl. Mater. Interfaces 2019, 11, 5682–5686. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, A.; Prabu, M.; Shanmugam, S. Porous LaCo1-xNixO3-delta Nanostructures as an Efficient Electrocatalyst for Water Oxidation and for a Zinc-Air Battery. ACS Appl. Mater. Interfaces 2016, 8, 6019–6031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, Y.; Ke, W.; Liang, Y.; Chao, Y.; Han, N.; Liang, P.; He, X. Enhanced cycling stability of La0.2Sr0.8CoO3−δ for oxygen evolution reaction via trace doping of Nb. Ceram. Int. 2022, 48, 36992–36999. [Google Scholar] [CrossRef]
- Wang, C.C.; Cheng, Y.; Ianni, E.; Jiang, S.P.; Lin, B. A highly active and stable La0.5Sr0.5Ni0.4Fe0.6O3-δ perovskite electrocatalyst for oxygen evolution reaction in alkaline media. Electrochim. Acta 2017, 246, 997–1003. [Google Scholar] [CrossRef]
- Feng, X.; Fan, Y.; Nomura, N.; Kikuchi, K.; Wang, L.; Jiang, W.; Kawasaki, A. Graphene promoted oxygen vacancies in perovskite for enhanced thermoelectric properties. Carbon 2017, 112, 169–176. [Google Scholar] [CrossRef]
- Yuan, R.-H.; He, Y.; He, W.; Ni, M.; Leung, M.K.H. Bifunctional electrocatalytic activity of La0.8Sr0.2MnO3-based perovskite with the A-site deficiency for oxygen reduction and evolution reactions in alkaline media. Appl. Energy 2019, 251, 113406. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, R.; Xu, W.; Pan, L.; Li, R.; Xiao, C.; Qiao, X. Ni-Doped La0.6Sr0.4CoO3 Perovskite as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions in Alkaline Media. Catalysts 2023, 13, 1366. https://doi.org/10.3390/catal13101366
Yuan R, Xu W, Pan L, Li R, Xiao C, Qiao X. Ni-Doped La0.6Sr0.4CoO3 Perovskite as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions in Alkaline Media. Catalysts. 2023; 13(10):1366. https://doi.org/10.3390/catal13101366
Chicago/Turabian StyleYuan, Ronghua, Weina Xu, Liquan Pan, Ruibin Li, Chuanying Xiao, and Xiaochang Qiao. 2023. "Ni-Doped La0.6Sr0.4CoO3 Perovskite as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions in Alkaline Media" Catalysts 13, no. 10: 1366. https://doi.org/10.3390/catal13101366
APA StyleYuan, R., Xu, W., Pan, L., Li, R., Xiao, C., & Qiao, X. (2023). Ni-Doped La0.6Sr0.4CoO3 Perovskite as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions in Alkaline Media. Catalysts, 13(10), 1366. https://doi.org/10.3390/catal13101366