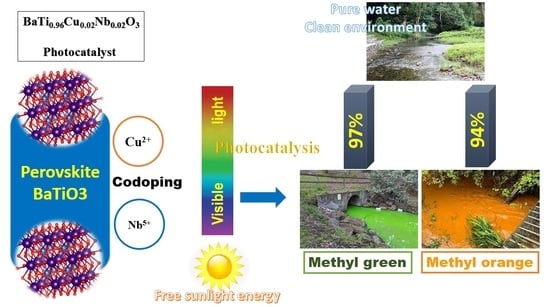
New BaTi0.96Cu0.02X0.02O3 (X = V, Nb) Photocatalysts for Dyes Effluent Remediation: Broad Visible Light Response
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction (XRD) Analysis
2.2. Scanning Electron Microscope (SEM) Analysis
2.3. Optical Properties and Band Gap Energy
2.4. Depollution Properties of the Photocatalysts
3. Preparation of Samples and Measurements
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belghiti, M.; Tanji, K.; El Mersly, L.; Lamsayety, I.; Ouzaouit, K.; Faqir, H.; Benzakour, I.; Rafqah, S.; Outzourhit, A. Fast and non-selective photodegradation of basic yellow 28, malachite green, tetracycline, and sulfamethazine using a nanosized ZnO synthesized from zinc ore. React. Kinet. Mech. Catal. 2022, 135, 2265–2278. [Google Scholar] [CrossRef]
- Dra, A.; Tanji, K.; Arrahli, A.; Iboustaten, E.; El Gaidoumi, A.; Kherchafi, A.; Benabdallah, A.C.; Kherbeche, A. Valorization of Oued Sebou natural sediments (Fez-Morocco Area) as adsorbent of methylene blue dye: Kinetic and thermodynamic study. Sci. World J. 2020, 2020, 2187129. [Google Scholar]
- Abid, M.Z.; Rafiq, K.; Rauf, A.; Shah, S.S.A.; Jin, R.; Hussain, E. Synergism of Co/Na in BiVO4 microstructures for visible-light driven degradation of toxic dyes in water. Nanoscale Adv. 2023, 5, 3247–3259. [Google Scholar] [CrossRef] [PubMed]
- Batra, V.; Kaur, I.; Pathania, D.; Sonu; Chaudhary, V. Efficient dye degradation strategies using green synthesized ZnO-based nanoplatforms: A review. Appl. Surf. Sci. Adv. 2022, 11, 100314. [Google Scholar] [CrossRef]
- Mzimela, N.; Tichapondwa, S.; Chirwa, E. Visible-light-activated photocatalytic degradation of rhodamine B using WO3 nanoparticles. RSC Adv. 2022, 12, 34652–34659. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Hu, H.; Zeng, G.; Li, C. Recovering hydrogen energy from photocatalytic treatment of pharmaceutical-contaminated water using Co3O4 modified {001}/{101}-TiO2 nanosheets. ACS EST Eng. 2021, 1, 603–611. [Google Scholar] [CrossRef]
- Ihsan, A.; Irshad, A.; Warsi, M.F.; Din, M.I.; Zulfiqar, S. NiFe2O4/ZnO nanoparticles and its composite with flat 2D rGO sheets for efficient degradation of colored and colorless effluents photocatalytically. Opt. Mater. 2022, 134, 113213. [Google Scholar] [CrossRef]
- Ighnih, H.; Haounati, R.; Malekshah, R.E.; Ouachtak, H.; Jada, A.; Addi, A.A. Photocatalytic degradation of RhB dye using hybrid nanocomposite BiOCl@Kaol under sunlight irradiation. J. Water Process. Eng. 2023, 54, 103925. [Google Scholar] [CrossRef]
- Pandit, M.A.; Billakanti, S.; Muralidharan, K. A simplistic approach for the synthesis of CuS-CdS heterostructure: A novel photo catalyst for oxidative dye degradation. J. Environ. Chem. Eng. 2020, 8, 103542. [Google Scholar] [CrossRef]
- Rajan, K.D.; Srinivasan, D.; Gotipamul, P.P.; Khanna, S.; Chidambaram, S.; Rathinam, M. Design of a novel ZnBi2O4/Bi2O3 type-II photo-catalyst via short term hydrothermal for enhanced degradation of organic pollutants. Mater. Sci. Eng. B 2022, 285, 115929. [Google Scholar] [CrossRef]
- Darabdhara, J.; Roy, S.; Ahmaruzzaman, M. Efficient photocatalytic degradation of an organic dye by the fabrication of a novel ternary composite based on zeolitic imidazolate framework via a facile in-situ synthetic approach. Inorg. Chem. Commun. 2023, 152, 110694. [Google Scholar] [CrossRef]
- Beura, R.; Rajendran, S.; Pinilla, M.A.G.; Thangadurai, P. Enhanced photo-induced catalytic activity of Cu ion doped ZnO—Graphene ternary nanocomposite for degrading organic dyes. J. Water Process. Eng. 2019, 32, 100966. [Google Scholar] [CrossRef]
- Jouali, A.; Salhi, A.; Aguedach, A.; Aarfane, A.; Ghazzaf, H.; Lhadi, E.K.; El krati, M.; Tahiri, S. Photo-catalytic degradation of methylene blue and reactive blue 21 dyes in dynamic mode using TiO2 particles immobilized on cellulosic fibers. J. Photochem. Photobiol. A 2019, 383, 112013. [Google Scholar] [CrossRef]
- Borah, D.; Saikia, P.; Sarmah, P.; Gogoi, D.; Rout, J.; Ghosh, N.N.; Bhattacharjee, C.R. Composition controllable alga-mediated green synthesis of covellite CuS nanostructure: An efficient photocatalyst for degradation of toxic dye. Inorg. Chem. Commun. 2022, 142, 109608. [Google Scholar] [CrossRef]
- Samanta, D.; Basnet, P.; Jha, S.; Chatterjee, S. Proficient route in synthesis of glucose stabilized Ag modified ZnS nanospheres for mechanistic understandings of commercially used dyes degradation. Inorg. Chem. Commun. 2022, 141, 109498. [Google Scholar] [CrossRef]
- Araujo, R.N.; Nascimento, E.P.; Firmino, H.C.T.; Macedo, D.A.; Neves, G.A.; Morales, M.A.; Menezes, R.R. α-Fe2O3 fibers: An efficient photocatalyst for dye degradation under visible light. J. Alloys Compd. 2021, 882, 160683. [Google Scholar] [CrossRef]
- Ismail, A.A.; Faisal, M.; Al-Haddad, A. Mesoporous WO3-graphene photocatalyst for photocatalytic degradation of Methylene Blue dye under visible light illumination. J. Environ. Sci. 2018, 66, 328–337. [Google Scholar] [CrossRef]
- Uma, P.I.; Shenoy, U.S.; Bhat, D.K. Doped BaTiO3 cuboctahedral nanoparticles: Role of copper in photocatalytic degradation of dyes. Appl. Surf. Sci. Adv. 2023, 15, 100408. [Google Scholar] [CrossRef]
- Ray, S.K.; Cho, J.; Hur, J. A critical review on strategies for improving efficiency of BaTiO3-based photocatalysts for wastewater treatment. J. Environ. Manag. 2021, 290, 112679. [Google Scholar] [CrossRef]
- Masekela, D.; Hintsho-Mbita, N.C.; Sam, S.; Yusuf, T.L.; Mabuba, N. Application of BaTiO3-based catalysts for piezocatalytic, photocatalytic and piezophotocatalytic degradation of organic pollutants and bacterial disinfection in wastewater: A comprehensive review. Arab. J. Chem. 2023, 16, 104473. [Google Scholar] [CrossRef]
- Elmahgary, M.G.; Mahran, A.M.; Ganoub, M.; Abdellatif, S.O. Optical investigation and computational modelling of BaTiO3 for optoelectronic devices applications. Sci. Rep. 2023, 13, 4761. [Google Scholar] [CrossRef] [PubMed]
- Bhat, D.K.; Bantawala, H.; Shenoy, U.S. Rhodium doping augments photocatalytic activity of barium titanate: Effect of electronic structure engineering. Nanoscale Adv. 2020, 2, 5688–5698. [Google Scholar] [CrossRef] [PubMed]
- Nageri, M.; Kumar, V. Manganese-doped BaTiO3 nanotube arrays for enhanced visible light photocatalytic applications. Mater. Chem. Phys. 2018, 213, 400–405. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Kumar, S.; Ahmed, J.; Ahamed, M.; Kumar, A. Influence of silver doping on the structure, optical and photocatalytic properties of Ag-doped BaTiO3 ceramics. Mater. Chem. Phys. 2021, 259, 124058. [Google Scholar] [CrossRef]
- Amaechi, I.C.; Youssef, A.H.; Kolhatkar, G.; Rawach, D.; Gomez-Yañez, C.; Claverie, J.P.; Sun, S.; Ruediger, A. Ultrafast microwave-assisted hydrothermal synthesis and photocatalytic behaviour of ferroelectric Fe3+-doped BaTiO3 nanoparticles under simulated sunlight. Catal. Today 2021, 360, 90–98. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, W.-S.; Kim, H.-T.; Yoon, D.-H. Properties of BaTiO3 synthesized from barium titanyl oxalate. Ceram. Int. 2009, 35, 2337–2342. [Google Scholar] [CrossRef]
- Khan, M.; Mishra, A.; Shukla, J.; Sharma, P. X-ray analysis of BaTiO3 ceramics by Williamson-Hall and size strain plot methods. Nanoscale Res. Lett. 2021, 16, 115. [Google Scholar]
- Salem, B.B.; Essalah, G.; Ameur, S.B.; Duponchel, B.; Guermazi, H.; Guermazia, S.; Leroy, G. Synthesis and comparative study of the structural and optical properties of binary ZnO-based composites for environmental applications. RSC Adv. 2023, 13, 6287–6303. [Google Scholar] [CrossRef] [PubMed]
- Kappadan, S.; Thomas, S.; Kalarikkal, N. Enhanced photocatalytic performance of BaTiO3/g-C3N4 heterojunction for the degradation of organic pollutants. Chem. Phys. Lett. 2021, 771, 138513. [Google Scholar] [CrossRef]
- Yang, F.; Yang, L.; Ai, C.; Xie, P.; Lin, S.; Wang, C.-Z.; Lu, X. Tailoring bandgap of perovskite BaTiO3 by transition metals Co-doping for visible-light photoelectrical applications: A first-principles study. Nanomaterials 2018, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.P.; Karanjekar, A.N.; Gaikwad, V.B.; Jain, G.H.; Shinde, S.D. Doping-induced diffused phase transition triggers gas-sensing performance of Sn-doped BaTiO3 nanostructures. Surf. Interface Anal. 2022, 54, 25–36. [Google Scholar] [CrossRef]
- Moriomoto, T.; Oka, R.; Minagawaa, K.; Masui, T. Novel near-infrared reflective black inorganic pigment based on cerium vanadate. RSC Adv. 2022, 12, 16570–16575. [Google Scholar] [CrossRef]
- Jiang, G.; Wei, Z.; Chen, H.; Du, X.; Li, L.; Liu, Y.; Huang, Q.; Chen, W. Preparation of novel carbon nanofibers with BiOBr and AgBr decoration for the photocatalytic degradation of rhodamine B. RSC Adv. 2015, 5, 30433–30437. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M. Enhanced photocatalytic and anticancer activity of Zn-doped BaTiO3 nanoparticles prepared through a green approach using Banana Peel extract. Catalysts 2023, 13, 985. [Google Scholar] [CrossRef]















| Samples | D (nm) | a Å | b Å | c Å | c/a | V Å3 |
|---|---|---|---|---|---|---|
| BaTiO3 | 71 | 3.9987 | 3.9987 | 4.0111 | 1.0031 | 64.1359 |
| BaTi0.96Cu0.04O3 | 61 | 4.0002 | 4.0002 | 4.0174 | 1.0038 | 64.2861 |
| BaTi0.96Cu0.02V0.02O3 | 58 | 4.0008 | 4.0008 | 4.0143 | 1.0033 | 64.2535 |
| BaTi0.96Cu0.02Nb0.02O3 | 56 | 4.0007 | 4.0007 | 4.0150 | 1.0035 | 64.2639 |
| Composition | Ba(NO3)2 (g) | TiO2 (g) | Cu(NO3)2·3H2O (g) | NH4VO3 (g) | NbCl5 (g) |
|---|---|---|---|---|---|
| BaTiO3 | 7.84 | 2.396 | - | - | - |
| BaTi0.96Cu0.04O3 | 7.84 | 2.30 | 0.29 | - | - |
| BaTi0.96Cu0.02V0.02O3 | 7.84 | 2.30 | 0.1449 | 0.07 | - |
| BaTi0.96Cu0.02Nb0.02O3 | 7.84 | 2.30 | 0.1449 | - | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsulaim, G.M. New BaTi0.96Cu0.02X0.02O3 (X = V, Nb) Photocatalysts for Dyes Effluent Remediation: Broad Visible Light Response. Catalysts 2023, 13, 1365. https://doi.org/10.3390/catal13101365
Alsulaim GM. New BaTi0.96Cu0.02X0.02O3 (X = V, Nb) Photocatalysts for Dyes Effluent Remediation: Broad Visible Light Response. Catalysts. 2023; 13(10):1365. https://doi.org/10.3390/catal13101365
Chicago/Turabian StyleAlsulaim, Ghayah M. 2023. "New BaTi0.96Cu0.02X0.02O3 (X = V, Nb) Photocatalysts for Dyes Effluent Remediation: Broad Visible Light Response" Catalysts 13, no. 10: 1365. https://doi.org/10.3390/catal13101365
APA StyleAlsulaim, G. M. (2023). New BaTi0.96Cu0.02X0.02O3 (X = V, Nb) Photocatalysts for Dyes Effluent Remediation: Broad Visible Light Response. Catalysts, 13(10), 1365. https://doi.org/10.3390/catal13101365