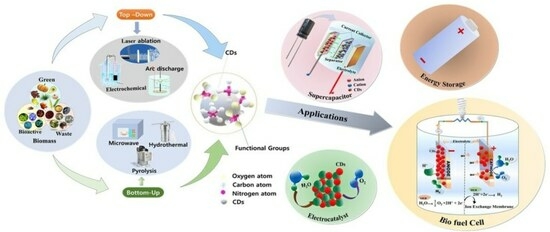
Comprehensive Review on Multifaceted Carbon Dot Nanocatalysts: Sources and Energy Applications
Abstract
:1. Introduction
2. Synthesis Techniques of Carbon Dots and Precursors
2.1. Green Biomass as Carbon Dot Precursors
2.2. Synthesis of Carbon Dots from Waste Biomass
2.3. Microorganisms and Bioactive Molecules as a Carbon Dot Precursor
3. Properties and Functionalization of Carbon Dots
4. Carbon Dots as Nanocatalysts in Energy Storage and Conversion
4.1. Biofuel Cells
4.2. Electrocatalysts for Energy Conversion
4.3. Supercapacitor
4.4. Photocatalysts
5. Economic Analysis
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bu, Y.; Kim, H.K.; Lee, J.S.; Jang, H.G.; Jeong, J.H.; Chun, S.W.; Sharma, M.; Kim, B.S. Improvement of biodegradable polymer film properties by incorporating functionalized few-layer graphene. Food Packag. Shelf Life 2023, 40, 101205. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Purkait, M.K. Chapter 16—Energy storage properties of nanomaterials. In Advances in Smart Nanomaterials and Their Applications; Husen, A., Siddiqi, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 337–350. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Chakraborty, A.; Purkait, M.K. 29—Extraction of clean energy from industrial wastewater using bioelectrochemical process. In Resource Recovery in Industrial Waste Waters; Sillanpää, M., Khadir, A., Gurung, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 601–620. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Sood, T.; Chakraborty, A.; Purkait, M.K. Reduced graphene oxide incorporated polyvinylidene fluoride/cellulose acetate proton exchange membrane for energy extraction using microbial fuel cells. J. Electroanal. Chem. 2022, 907, 115890. [Google Scholar] [CrossRef]
- Singh, A.; Kushwaha, A.; Goswami, S.; Tripathi, A.; Bhasney, S.M.; Goswami, L.; Hussain, C.M. Roadmap from microalgae to biorefinery: A circular bioeconomy approach. In Emerging Trends to Approaching Zero Waste: Environmental and Social Perspectives; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Sustainable synthesis of multifunctional carbon dots using biomass and their applications: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105802. [Google Scholar] [CrossRef]
- Sharma, M.; Chakraborty, A.; Kuttippurath, J.; Yadav, A.K. Potential Power Production from Salinity Gradient at the Hooghly Estuary System. Innov. Energy Res. 2018, 7, 210. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2015, 183, 519–542. [Google Scholar] [CrossRef]
- Wu, S.; Li, W.; Zhou, W.; Zhan, Y.; Hu, C.; Zhuang, J.; Zhang, H.; Zhang, X.; Lei, B.; Liu, Y. Large-Scale One-Step Synthesis of Carbon Dots from Yeast Extract Powder and Construction of Carbon Dots/PVA Fluorescent Shape Memory Material. Adv. Opt. Mater. 2018, 6, 1701150. [Google Scholar] [CrossRef]
- Pham, P.V.; Bodepudi, S.C.; Shehzad, K.; Liu, Y.; Xu, Y.; Yu, B.; Duan, X. 2D Heterostructures for Ubiquitous Electronics and Optoelectronics: Principles, Opportunities, and Challenges. Chem. Rev. 2022, 122, 6514–6613. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S.; Zheng, L.; Liu, Z.; Zhang, G.; Wu, H.; Wang, B.; Liu, Z.; Jin, Z.; Wang, G. Si-Based NIR Tunneling Heterojunction Photodetector With Interfacial Engineering and 3D-Graphene Integration. IEEE Electron Device Lett. 2022, 43, 1818–1821. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Fan, S.; Li, J.; Mu, J.; Qin, M.; Wang, L.; Gan, G.; Tadé, M.; Liu, S. The bioelectrochemical synthesis of high-quality carbon dots with strengthened electricity output and excellent catalytic performance. Nanoscale 2019, 11, 4428–4437. [Google Scholar] [CrossRef]
- Mocci, F.; Engelbrecht, L.d.V.; Olla, C.; Cappai, A.; Casula, M.F.; Melis, C.; Stagi, L.; Laaksonen, A.; Carbonaro, C.M. Carbon Nanodots from an In Silico Perspective. Chem. Rev. 2022, 122, 13709–13799. [Google Scholar] [CrossRef]
- Langer, M.; Paloncýová, M.; Medveď, M.; Pykal, M.; Nachtigallová, D.; Shi, B.; Aquino, A.J.A.; Lischka, H.; Otyepka, M. Progress and challenges in understanding of photoluminescence properties of carbon dots based on theoretical computations. Appl. Mater. Today 2021, 22, 100924–100951. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis-A short review. Carbon Trends 2021, 3, 32. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Wu, G.; Gao, Y.; Zhao, D.; Ling, P.; Gao, F. Methanol/Oxygen Enzymatic Biofuel Cell Using Laccase and NAD+-Dependent Dehydrogenase Cascades as Biocatalysts on Carbon Nanodots Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 40978–40986. [Google Scholar] [CrossRef] [PubMed]
- Darinel, S.; Landa, T.; Kumar, N.; Bogireddy, R.; Kaur, I.; Batra, V.; Agarwal, V. Heavy metal ion detection using green precursor derived carbon dots. iScience 2022, 25, 103816. [Google Scholar]
- Devadas, B.; Imae, T. Effect of Carbon Dots on Conducting Polymers for Energy Storage Applications. ACS Sustain. Chem. Eng. 2018, 6, 127–134. [Google Scholar] [CrossRef]
- Duarah, P.; Das, P.P.; Sharma, M.; Purkait, M.K. Recent Advances in Dye Removal Technologies by Designer Biochar. In Designer Biochar Assisted Bioremediation of Industrial Effluents; CRC Press: Boca Raton, FL, USA, 2022; pp. 223–239. [Google Scholar]
- Essner, J.B.; Kist, J.A.; Polo-Parada, L.; Baker, G.A. Artifacts and Errors Associated with the Ubiquitous Presence of Fluorescent Impurities in Carbon Nanodots. Chem. Mater. 2018, 30, 1878–1887. [Google Scholar] [CrossRef]
- Rizvi, S.; Singh, A.; Gupta, S.K. A parametric study using Box-Behnken design for melanoidin removal via Cu-impregnated activated carbon prepared from waste leaves biomass. Appl. Water Sci. 2022, 12, 81. [Google Scholar] [CrossRef]
- Rizvi, S.; Goswami, L.; Gupta, S.K. A holistic approach for melanoidin removal via Fe-impregnated activated carbon prepared from Mangifera indica leaves biomass. Bioresour. Technol. Rep. 2020, 12, 100591. [Google Scholar] [CrossRef]
- Sharma, M.; Mondal, P.; Sontakke, A.D.; Chakraborty, A.; Purkait, M.K. High performance graphene-oxide doped cellulose acetate based ion exchange membrane for environmental remediation applications. Int. J. Environ. Anal. Chem. 2021, 1–22. [Google Scholar] [CrossRef]
- Neto, S.A.; de Andrade, A.R. New energy sources: The enzymatic biofuel cell. J. Braz. Chem. Soc. 2013, 24, 1891–1912. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S.M.; Xiong, H.F.; Alwahibi, M.S.; Niu, X. Biosynthesis of copperoxide nanoparticles using Abies spectabilis plant extract and analyzing its antinociceptive and anti-inflammatory potency in various mice models. Arab. J. Chem. 2020, 13, 6995–7006. [Google Scholar] [CrossRef]
- Lecroy, G.E.; Messina, F.; Sciortino, A.; Bunker, C.E.; Wang, P.; Fernando KA, S.; Sun, Y.P. Characteristic Excitation Wavelength Dependence of Fluorescence Emissions in Carbon “quantum” Dots. J. Phys. Chem. C 2017, 121, 28180–28186. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Qiu, J.; Sun, Y.P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337. [Google Scholar] [CrossRef]
- Arul, V.; Edison TN, J.I.; Lee, Y.R.; Sethuraman, M.G. Biological and catalytic applications of green synthesized fluorescent N-doped carbon dots using Hylocereus undatus. J. Photochem. Photobiol. B Biol. 2017, 168, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Ortega-Liebana, M.C.; Encabo-Berzosa, M.M.; Casanova, A.; Pereboom, M.D.; Alda, J.O.; Hueso, J.L.; Santamaria, J. Upconverting Carbon Nanodots from Ethylenediaminetetraacetic Acid (EDTA) as Near-Infrared Activated Phototheranostic Agents. Chem.—A Eur. J. 2019, 25, 5539–5546. [Google Scholar] [CrossRef]
- Ventrella, A.; Camisasca, A.; Fontana, A.; Giordani, S. Synthesis of green fluorescent carbon dots from carbon nano-onions and graphene oxide. RSC Adv. 2020, 10, 36404–36412. [Google Scholar] [CrossRef]
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-Based Carbon Dots: Current Development and Future Perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef]
- Sharker, S.M.d.; Do, M. Nanoscale Carbon-Polymer Dots for Theranostics and Biomedical Exploration. J. Nanotheranostics 2021, 2, 118–130. [Google Scholar] [CrossRef]
- Hoang, V.C.; Dave, K.; Gomes, V.G. Carbon quantum dot-based composites for energy storage and electrocatalysis: Mechanism, applications and future prospects. Nano Energy 2019, 66, 104093. [Google Scholar] [CrossRef]
- Kalanidhi, K.; Nagaraaj, P. Facile and Green synthesis of fluorescent N-doped carbon dots from betel leaves for sensitive detection of Picric acid and Iron ion. J. Photochem. Photobiol. A Chem. 2021, 418, 113369. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Wang, C.; Zheng, H.; Huang, Z.; Chen, D.; Xie, H. Photoluminescent carbon dots derived from sugarcane molasses: Synthesis, properties, and applications. RSC Adv. 2017, 7, 47840–47847. [Google Scholar] [CrossRef]
- Venkatesan, G.; Rajagopalan, V.; Chakravarthula, S.N. Boswellia ovalifoliolata bark extract derived carbon dots for selective fluorescent sensing of Fe3+. J. Environ. Chem. Eng. 2019, 7, 103013. [Google Scholar] [CrossRef]
- Jiang, Z.; Guan, L.; Xu, X.; Wang, E.; Wang, C. Applications of Carbon Dots in Electrochemical Energy Storage. ACS Appl. Electron. Mater. 2022, 4, 5144–5164. [Google Scholar] [CrossRef]
- Arsalani, N.; Ghadimi, L.S.; Ahadzadeh, I.; Tabrizi, A.G.; Nann, T. Green Synthesized Carbon Quantum Dots/Cobalt Sulfide Nanocomposite as Efficient Electrode Material for Supercapacitors. Energy Fuels 2021, 35, 9635–9645. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon quantum dots for advanced electrocatalysis. J. Energy Chem. 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Shen, D.; Zhu, L.; Wu, C.; Gu, S. State-of-the-art on the preparation, modification, and application of biomass-derived carbon quantum dots. Ind. Eng. Chem. Res. 2020, 59, 22017–22039. [Google Scholar] [CrossRef]
- Cai, P.; Wang, X.; Seo, H.J. Excitation power dependent optical temperature behaviors in Mn4+ doped oxyfluoride Na2WO2F4. Phys. Chem. Chem. Phys. 2018, 20, 2028–2035. [Google Scholar] [CrossRef]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A. Carbon Dots from Sugars and Ascorbic Acid: Role of the Precursors on Morphology, Properties, Toxicity, and Drug Uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.K.K.; Tan, H.L.; Lim, Y.P.; So’aib, M.S.; Bakar, N.F.A. A Review on Multifunctional Carbon-Dots Synthesized From Biomass Waste: Design/ Fabrication, Characterization and Applications. Front. Energy Res. 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Jin, S.; Zhou, Y.; Ji, Q.; Lan, H.; Liu, H.; Qu, J. Graphitic N in nitrogen-Doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction. Carbon 2020, 163, 154–161. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, C.; Zhou, Y.; Zhao, T.; Leblanc, R.M. Polyethylene Glycol (PEG) Derived Carbon Dots: Preparation and Applications. Appl. Mater. Today 2020, 20, 100677. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.; Chen, X.; Wang, D.; Cai, Y. Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C 2017, 76, 856–864. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Zhou, Q.; Zhou, Q.; Zhang, Y.; Zhu, L. Polycyclic musks in the environment: A review of their concentrations and distribution, ecological effects and behavior, current concerns and future prospects. Crit. Rev. Environ. Sci. Technol. 2021, 51, 323–377. [Google Scholar] [CrossRef]
- Oskueyan, G.; Mansour Lakouraj, M.; Mahyari, M. Fabrication of polyaniline–carrot derived carbon dots/polypyrrole–graphene nanocomposite for wide potential window supercapacitor. Carbon Lett. 2021, 31, 269–276. [Google Scholar] [CrossRef]
- Zhao, C.; Jiao, Y.; Hu, F.; Yang, Y. Green synthesis of carbon dots from pork and application as nanosensors for uric acid detection. Spectrochim. Acta—A Mol. Biomol. Spectrosc. 2018, 190, 360–367. [Google Scholar] [CrossRef]
- Singh, A.; Kushwaha, A.; Sen, S.; Goswami, S.; Katiyar, S.; Kumar, A.; Borah, S.N.; Goswami, L.; Hussain, C.M. Recent advancement in microwave-assisted pyrolysis for biooil production. In Waste-to-Energy Approaches Towards Zero Waste: Interdisciplinary Methods of Controlling Waste; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Singh, A.; Saha, P.; Choi, Y.; Maharana, M.; Patil, S.V.; Kim, B.S. Nano-Biochar as a Sustainable Catalyst for Anaerobic Digestion: A Synergetic Closed-Loop Approach. Catalysts 2022, 12, 186. [Google Scholar] [CrossRef]
- Jana, J.; Ngo, Y.L.T.; Chung, J.S.; Hur, S.H. Contribution of carbon dot nanoparticles in electrocatalysis: Development in energy conversion process. J. Electrochem. Sci. Technol. 2020, 11, 220–237. [Google Scholar] [CrossRef]
- Pankaj, A.; Tewari, K.; Singh, S.; Singh, S.P. Waste candle soot derived nitrogen doped carbon dots based fluorescent sensor probe: An efficient and inexpensive route to determine Hg(II) and Fe(III) from water. J. Environ. Chem. Eng. 2018, 6, 5561–5569. [Google Scholar] [CrossRef]
- Thakur, A.; Devi, P.; Saini, S.; Jain, R.; Sinha, R.K.; Kumar, P. Citrus limetta Organic Waste Recycled Carbon Nanolights: Photoelectro Catalytic, Sensing, and Biomedical Applications. ACS Sustain. Chem. Eng. 2019, 7, 502–512. [Google Scholar] [CrossRef]
- El-Sayyad, G.S.; Mosallam, F.M.; El-Batal, A.I. One-pot green synthesis of magnesium oxide nanoparticles using Penicillium chrysogenum melanin pigment and gamma rays with antimicrobial activity against multidrug-resistant microbes. Adv. Powder Technol. 2018, 29, 2616–2625. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Chen, Z. Bacteria-derived carbon dots inhibit biofilm formation of Escherichia coli without affecting cell growth. Front. Microbiol. 2018, 9, 259. [Google Scholar] [CrossRef]
- Manivasagan, P.; Nam, S.Y.; Oh, J. Marine microorganisms as potential biofactories for synthesis of metallic nanoparticles. Crit. Rev. Microbiol. 2016, 42, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.A.; Kumar, V.B.; Fixler, D.; Dubinsky, Z.; Gedanken, A.; Iluz, D. Nitrogen-doped carbon dots prepared from bovine serum albumin to enhance algal astaxanthin production. Algal Res. 2017, 23, 161–165. [Google Scholar] [CrossRef]
- Bakhshi, M.; Mohammad, S.; Hosseini, R.; Rahimi, M.; Hosseini, M.R. Green Synthesis of CdS Nanoparticles Using Metabolites of Bacillus Licheniformis Using AASX (Air-Assisted Solvent Extraction) for Dilute Solution View Project Green Synthesis of CdS Nanoparticles Using Metabolites of Bacillus Licheniformis. 2016. Available online: https://www.researchgate.net/publication/308162960 (accessed on 1 October 2023).
- Gong, X.; Li, Z.; Hu, Q.; Zhou, R.; Shuang, S.; Dong, C. N,S,P Co-Doped Carbon Nanodot Fabricated from Waste Microorganism and Its Application for Label-Free Recognition of Manganese(VII) and l -Ascorbic Acid and AND Logic Gate Operation. ACS Appl. Mater. Interfaces 2017, 9, 38761–38772. [Google Scholar] [CrossRef] [PubMed]
- Ganjkhanlou, Y.; Maris, J.E.; Koek, J.; Riemersma, R.; Weckhuysen, B.M.; Meirer, F. Dual Fluorescence in Glutathione-Derived Carbon Dots Revisited. J. Phys. Chem. C 2022, 126, 2720–2727. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.; Iqbal, M.; Faisal, A.; Junjua, A.M.; Hussain, I.; Hussain, S.Z.; Ghramh, H.A.; Khan, K.A.; Janjua, H.A. Hydrothermal synthesis of carbon nanodots from bovine gelatin and PHM3 microalgae strain for anticancer and bioimaging applications. Nanoscale Adv. 2019, 1, 2924–2936. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Wang, Z.; Yun, S.; Wang, X.; Wang, C.; Si, Y.; Zhang, Y.; Xu, H. Aloe peel-derived honeycomb-like bio-based carbon with controllable morphology and its superior electrochemical properties for new energy devices. Ceram. Int. 2019, 45, 4208–4218. [Google Scholar] [CrossRef]
- Chatzimarkou, A.; Chatzimitakos, T.G.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Stalikas, C.D. Selective FRET-based sensing of 4-nitrophenol and cell imaging capitalizing on the fluorescent properties of carbon nanodots from apple seeds. Sens. Actuators B Chem. 2018, 258, 1152–1160. [Google Scholar] [CrossRef]
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst 2015, 140, 4260–4269. [Google Scholar] [CrossRef] [PubMed]
- Meiyazhagan, A.; Aliyan, A.; Ayyappan, A.; Moreno-Gonzalez, I.; Susarla, S.; Yazdi, S.; Cuanalo-Contreras, K.; Khabashesku, V.N.; Vajtai, R.; Martí, A.A.; et al. Soft-Lithographic Patterning of Luminescent Carbon Nanodots Derived from Collagen Waste. ACS Appl. Mater. Interfaces 2018, 10, 36275–36283. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, L. One-step sonochemical synthesis of versatile nitrogen-doped carbon quantum dots for sensitive detection of Fe2+ ions and temperature in vitro. Mater. Sci. Eng. C 2019, 101, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhao, N.; Yan, L.; Zhong, P.; Nguyen, V.C.; Le, P.H. Double-pulse femtosecond laser ablation for synthesis of ultrasmall carbon nanodots. Mater. Res. Express 2019, 7, 015606. [Google Scholar] [CrossRef]
- Yao, B.; Huang, H.; Liu, Y.; Kang, Z. Carbon Dots: A Small Conundrum. Trends Chem. 2019, 1, 235–246. [Google Scholar] [CrossRef]
- Kumar, A.; Narayanan, S.S.; Thapa, B.S.; Pandit, S.; Pant, K.; Mukhopadhyay, A.K.; Peera, S.G. Application of Low-Cost Plant-Derived Carbon Dots as a Sustainable Anode Catalyst in Microbial Fuel Cells for Improved Wastewater Treatment and Power Output. Catalysts 2022, 12, 1580. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Progress and insights in the application of mxenes as new 2d nanomaterials suitable for biosensors and biofuel cell design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2018, 30, 1870002. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Arcudi, F.; Đorđević, L.; Prato, M. Rationally Designed Carbon Nanodots towards Pure White-Light Emission. Angew. Chem. 2017, 129, 4234–4237. [Google Scholar] [CrossRef]
- Dias, C.; Vasimalai, N.; Sárria, M.P.; Pinheiro, I.; Vilas-Boas, V.; Peixoto, J.; Espiña, B. Biocompatibility and bioimaging potential of fruit-based carbon dots. Nanomaterials 2019, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, C.; Li, Z.; Liu, J.; Xiao, X.; Li, D.; Chen, C.; Yu, M.; Feng, Y. Accelerating the extracellular electron transfer of Shewanella oneidensis MR-1 by carbon dots: The role of carbon dots concentration. Electrochim. Acta 2022, 421, 140490. [Google Scholar] [CrossRef]
- Tripathi, B.; Pandit, S.; Sharma, A.; Chauhan, S.; Mathuriya, A.S.; Dikshit, P.K.; Gupta, P.K.; Singh, R.C.; Sahni, M.; Pant, K.; et al. Modification of Graphite Sheet Anode with Iron (II, III) Oxide-Carbon Dots for Enhancing the Performance of Microbial Fuel Cell. Catalysts 2022, 12, 1040. [Google Scholar] [CrossRef]
- Guo, D.; Wei, H.-F.; Song, R.-B.; Fu, J.; Lu, X.; Jelinek, R.; Min, Q.; Zhang, J.-R.; Zhang, Q.; Zhu, J.-J. N,S-doped carbon dots as dual-functional modifiers to boost bio-electricity generation of individually-modified bacterial cells. Nano Energy 2019, 63. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y.K. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 2013, 7, 6858–6867. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, Z.W.; Pan, S.T.; He, Z.X.; Zhang, X.; Qiu, J.X.; Duan, W.; Yang, T.; Zhou, S.F. Graphene quantum dots induce apoptosis, autophagy, and inflammatory response via p38 mitogen-activated protein kinase and nuclear factor-κB mediated signaling pathways in activated THP-1 macrophages. Toxicology 2015, 327, 62–76. [Google Scholar] [CrossRef]
- Sharma, M.; Pramanik, A.; Bhowmick, G.D.; Tripathi, A.; Ghangrekar, M.M.; Pandey, C.; Kim, B.-S. Premier, Progress and Prospects in Renewable Hydrogen Generation: A Review. Fermentation 2023, 9, 537. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent advances in anodes for microbial fuel cells: An overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Gao, X.; Qiu, S.; Lin, Z.; Xie, X.; Yin, W.; Lu, X. Carbon-Based Composites as Anodes for Microbial Fuel Cells: Recent Advances and Challenges. ChemPlusChem 2021, 86, 1322–1341. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Calzoni, E.; Cesaretti, A.; di Michele, A.; Emiliani, C.; Gammaitoni, L.; Sisani, E. Enzymatic fuel cell technology for energy production from bio-sources. AIP Conf. Proc. 2019, 2191, 020014. [Google Scholar] [CrossRef]
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers. Nat. Commun. 2018, 9, 4479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gao, Y.; Sun, J.; Gao, F. Mediatorless glucose biosensor and direct electron transfer type glucose/air biofuel cell enabled with carbon nanodots. Anal. Chem. 2015, 87, 2615–2622. [Google Scholar] [CrossRef]
- De Poulpiquet, A.; Ciaccafava, A.; Lojou, E. New trends in enzyme immobilization at nanostructured interfaces for efficient electrocatalysis in biofuel cells. Electrochim. Acta 2014, 126, 104–114. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal-Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, T.; Tao, K.; Chang, H. Generating Electricity on Chips: Microfluidic Biofuel Cells in Perspective. Ind. Eng. Chem. Res. 2018, 57, 2746–2758. [Google Scholar] [CrossRef]
- Haque, S.U.; Nasar, A.; Duteanu, N.; Pandey, S. Carbon based-nanomaterials used in biofuel cells—A review. Fuel 2022, 331, 125634. [Google Scholar] [CrossRef]
- Cosnier, S.; Holzinger, M.; Goff, A. Recent advances in carbon nanotube-based enzymatic fuel cells. Front. Bioeng. Biotechnol. 2014, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, S.; Yang, B. Carbon-Dot-Enhanced Electrocatalytic Hydrogen Evolution. Acc. Mater. Res. 2022, 3, 319–330. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, G.; Arya, S.K. Biofuel cell nanodevices. Int. J. Hydrogen Energy 2021, 46, 3270–3288. [Google Scholar] [CrossRef]
- Voiry, D.; Shin, H.S.; Loh, K.P.; Chhowalla, M. Low-dimensional catalysts for hydrogen evolution and CO2 reduction. Nat. Rev. Chem. 2018, 2, 0105. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Guo, S.; Liu, Y.; Kang, Z. A nickel nanoparticle/carbon quantum dot hybrid as an efficient electrocatalyst for hydrogen evolution under alkaline conditions. J. Mater. Chem. A 2015, 3, 18598–18604. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Zhou, M.; Yang, K.; Yang, S.; Ma, X.; Yu, C.; Xie, Y.; Huang, W.; Fan, Q. A Novel Approach to Synthesize Nitrogen-Deficient g-C3N4 for the Enhanced Photocatalytic Contaminant Degradation and Electrocatalytic Hydrogen Evolution. Nano 2020, 15, 2050006. [Google Scholar] [CrossRef]
- Nazir, R.; Kumar, A.; Saleh Saad, M.A.; Ashok, A. Synthesis of hydroxide nanoparticles of Co/Cu on carbon nitride surface via galvanic exchange method for electrocatalytic CO2 reduction into formate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124835. [Google Scholar] [CrossRef]
- Xiao, J.; Momen, R.; Liu, C. Application of carbon quantum dots in supercapacitors: A mini review. Electrochem. Commun. 2021, 132, 107143. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, K.; Wang, L.; Lee, C.S. Oxygen/nitrogen-related surface states controlled carbon nanodots with tunable full-color luminescence: Mechanism and bio-imaging. Carbon 2020, 160, 298–306. [Google Scholar] [CrossRef]
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-based quantum dots for supercapacitors: Recent advances and future challenges. Nanomaterials 2021, 11, 91. [Google Scholar] [CrossRef]
- Xu, L.; Dun, X.; Zou, J.; Li, Y.; Jia, M.; Cui, L.; Gao, J.; Jin, X. Graphene Hydrogel Decorated with N, O Co-Doped Carbon Dots for Flexible Supercapacitor Electrodes. J. Electrochem. Soc. 2018, 165, A2217–A2224. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Xin, S.; Yang, Z.; Li, Y.; Zhang, D.; Yao, P. Facile Synthesis of MoS2/Reduced Graphene Oxide@Polyaniline for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 21373–21380. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Wu, X.; Meng, M.; Zhu, X.; Yang, L.; Chu, P.K. Photothermal Contribution to Enhanced Photocatalytic Performance of Graphene-Based Nanocomposites. ACS Nano 2014, 8, 9304–9310. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shi, Y.; Chen, Z.; Sun, X.; Yuan, H.; Guo, F.; Shi, W. Photothermal effect of carbon dots for boosted photothermal-assisted photocatalytic water/seawater splitting into hydrogen. Chem. Eng. J. 2023, 453, 139834. [Google Scholar] [CrossRef]



| No. | Precursor | Image | Size | Quantum Yield | Synthesis Method | Type of CDs | Application | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Onion waste |  | 0.21 nm | 28% | Autoclave | CDs | Antimicrobial activity against HEK-293 cells, HeLa cells, Fe2+ ion probe detection | [67] |
| 2 | Aloe peel |  | <10 nm | NA | Hydrothermal | N-CDs | Electrode | [68] |
| 3 | Betel leaf |  | <10 nm | NA | Hydrothermal | N-CDs | Picric acid and Fe ion detector probe | [37] |
| 4 | Sugarcane bagasse |  | 1.9 nm | 5.80% | Hydrothermal | GQDs | Bioimaging (MCF-7 cells) | [38] |
| 5 | Apple seeds |  | <10 nm | NA | Pyrolysis | CDs | Detection of 4-nitrophenol, bioimaging | [69] |
| 6 | Coriander leaves |  | <10 nm | NA | Hydrothermal | CDs | Fe3+ detection, bioimaging for lung normal (L-132) and cancer (A549) cell lines | [70] |
| 7 | Citrus limetta |  | 4~7 nm | 63.30% | Pyrolysis | G-CDs | Photoelectrochemical water splitting, dye removal (MB), Fe (III) ions sensor, anti-bactericidal activity | [58] |
| 8 | Carrot |  | <10 nm | NA | Hydrothermal | Polymer CDs | Supercapacitor | [52] |
| 9 | Animal skin waste (Collagen) |  | <10 nm | NA | Hydrothermal | CDs, biofilm | Nanophotonics | [71] |
| 10 | Dopamine |  | 2–4 nm | NA | Sonochemical | CQDs | Fe2+ detection, nanothermometer to sense temperature both in water and in cells | [72] |
| 11 | Ultra-pure water impregnated by TiO2 |  | 3–6 nm | NA | Hydrothermal | TiO2/CDs nanohybrid | Photocatalytic activity, peroxidase mimicking activity | [73] |
| 12 | Candle soot |  | 2–5 nm | NA | Hydrothermal | N-CDs | Hg and Fe ions probe detector | [57] |
| 13 | Citric acid with Ppy * and PANI * |  | <10 nm | NA | Hydrothermal | Ppy-CDs | Electrode | [20] |
| 14 | Graphite Powders |  | 1 nm | NA | Laser ablation | N-CDs | Catalytic and biosensor | [74] |
| 15 | Penicillium chrysogenum |  | 10.28 nm | NA | Gamma-ray irradiation | CDs | Antimicrobial agents against Enterococcus faecalis, Candida albicans, and Klebsiella pneumoniae | [59] |
| Catalyst Name | Synthesis Method | Size (nm) | Current Density (mA/cm2) | Applications | References |
|---|---|---|---|---|---|
| Graphitic N | Hydrogel | 3–5 | 1.62 | ORR, HER, photocatalysis | [47] |
| Nitrogen-deficient g-C3N4 | Hydrothermal | <10 | 10 | HER | [102] |
| C3N4/(Co(OH)2/Cu(OH)2 | Microwave-assisted | 0.27 | 8.9 | CO2 reduction | [103] |
| Ni-CDs | Electrochemical | 2–5 | 0.23 | HER | [101] |
| Polyaniline Carrot derived-CDs | Hydrothermal | 6–8 | 5 A/g | Window supercapacitor | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Kafle, S.R.; Sharma, M.; Kim, B.S. Comprehensive Review on Multifaceted Carbon Dot Nanocatalysts: Sources and Energy Applications. Catalysts 2023, 13, 1446. https://doi.org/10.3390/catal13111446
Singh A, Kafle SR, Sharma M, Kim BS. Comprehensive Review on Multifaceted Carbon Dot Nanocatalysts: Sources and Energy Applications. Catalysts. 2023; 13(11):1446. https://doi.org/10.3390/catal13111446
Chicago/Turabian StyleSingh, Anju, Saroj Raj Kafle, Mukesh Sharma, and Beom Soo Kim. 2023. "Comprehensive Review on Multifaceted Carbon Dot Nanocatalysts: Sources and Energy Applications" Catalysts 13, no. 11: 1446. https://doi.org/10.3390/catal13111446
APA StyleSingh, A., Kafle, S. R., Sharma, M., & Kim, B. S. (2023). Comprehensive Review on Multifaceted Carbon Dot Nanocatalysts: Sources and Energy Applications. Catalysts, 13(11), 1446. https://doi.org/10.3390/catal13111446