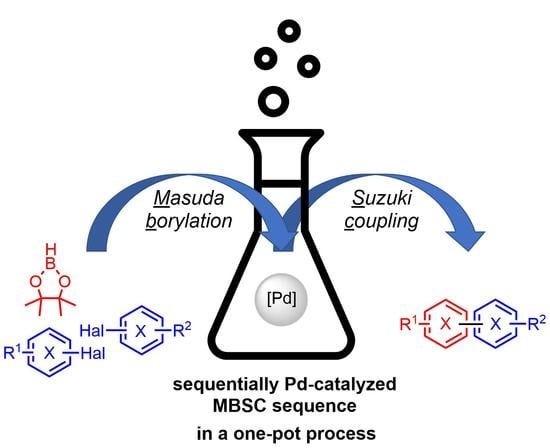
Masuda Borylation–Suzuki Coupling (MBSC) Sequence: A One-Pot Process to Access Complex (hetero)Biaryls
Abstract
:1. Introduction
2. Synthesis
2.1. Baudoin’s First One-Pot MBSC Synthesis of ortho,ortho’-Biaryls
2.2. Queiroz’s One-Pot MBSC Sequence with Benzothiophene Substrates
2.3. Levacher’s One-Pot MBSC Sequence with Naphthyl Substrates
2.4. Colobert’s One-Pot MBSC Biaryl Synthesis
2.5. Chai’s and Huleatt’s One-Pot MBSC Biindole Synthesis
2.6. Müller’s Generalized One-Pot MBSC Synthesis of bi(hetero)Aryls
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Biteau, N.G.; Roy, V.; Nicolas, C.; Becker, H.F.; Lambry, J.-C.; Myllykallio, H.; Agrofoglio, L.A. Synthesis and Structure–Activity Relationship Studies of Pyrido [1,2-e]Purine-2,4(1H,3H)-Dione Derivatives Targeting Flavin-Dependent Thymidylate Synthase in Mycobacterium tuberculosis. Molecules 2022, 27, 6216. [Google Scholar] [CrossRef] [PubMed]
- Iwan, D.; Kamińska, K.; Wojaczyńska, E.; Psurski, M.; Wietrzyk, J.; Daszkiewicz, M. Biaryl Sulfonamides Based on the 2-Azabicycloalkane Skeleton—Synthesis and Antiproliferative Activity. Materials 2020, 13, 5010. [Google Scholar] [CrossRef]
- Tajuddeen, N.; Feineis, D.; Ihmels, H.; Bringmann, G. The Stereoselective Total Synthesis of Axially Chiral Naphthylisoquinoline Alkaloids. Acc. Chem. Res. 2022, 55, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Medici, F.; Resta, S.; Puglisi, A.; Rossi, S.; Raimondi, L.; Benaglia, M. Electrochemical Organic Synthesis of Electron-Rich Biaryl Scaffolds: An Update. Molecules 2021, 26, 6968. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. Suzuki–Miyaura Cross-Coupling Reactions in Aqueous Media: Green and Sustainable Syntheses of Biaryls. ChemSusChem 2010, 3, 502–522. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, H.; Chen, Z.; Gao, J.; Yang, M.; Yuan, Z.; Li, X. A Novel Utilization of Water Extract of Suaeda Salsa in the Pd/C Catalyzed Suzuki–Miyaura Coupling Reaction. Molecules 2022, 27, 6623. [Google Scholar] [CrossRef]
- Choy, P.Y.; Wong, S.M.; Kapdi, A.; Kwong, F.Y. Recent developments in palladium-catalysed non-directed coupling of (hetero)arene C–H bonds with C–Z (Z = B, Si, Sn, S, N, C, H) bonds in bi(hetero)aryl synthesis. Organic Chemistry Frontiers 2018, 5, 288–321. [Google Scholar] [CrossRef]
- Cook, X.A.F.; de Gombert, A.; McKnight, J.; Pantaine, L.R.E.; Willis, M.C. The 2-Pyridyl Problem: Challenging Nucleophiles in Cross-Coupling Arylations. Angew. Chem. Int. Ed. 2021, 60, 11068–11091. [Google Scholar] [CrossRef]
- Buskes, M.J.; Blanco, M.-J. Impact of Cross-Coupling Reactions in Drug Discovery and Development. Molecules 2020, 25, 3493. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, W.; Tang, W. Palladium-catalyzed cross-couplings in the synthesis of agrochemicals. Advanced Agrochem 2022, 1, 125–138. [Google Scholar] [CrossRef]
- May, L.; Daniel, S.; Müller, T.J.J. Diversity-oriented approach to functional thiophene dyes by Suzuki coupling-lithiation one-pot sequences. Org. Chem. Front. 2020, 7, 329–339. [Google Scholar] [CrossRef]
- Miyaura, N.; Yanagi, T.; Suzuki, A. The Palladium-Catalyzed Cross-Coupling Reaction of Phenylboronic Acid with Haloarenes in the Presence of Bases. Synth. Commun. 1981, 11, 513–519. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Roy, D.; Uozumi, Y. Recent Advances in Palladium-Catalyzed Cross-Coupling Reactions at ppm to ppb Molar Catalyst Loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.M.; Friedrich, H.B. The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions. Molecules 2018, 23, 1676. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct C-C Bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef]
- Franz, A.W.; Müller, T.J.J. Facile Synthesis of Functionalized Oligophenothiazines via One-Pot Bromine-Lithium Exchange-Borylation-Suzuki Coupling (BLEBS). Synthesis 2008, 2008, 1121–1125. [Google Scholar] [CrossRef]
- Wiefermann, J.; Schmeinck, P.; Ganter, C.; Müller, T.J.J. Highly Deep-Blue Luminescent Twisted Diphenylamino Terphenyl Emitters by Bromine-Lithium Exchange Borylation-Suzuki Sequence. Chem. Eur. J. 2022, 28, e202200576. [Google Scholar] [CrossRef]
- Xu, L. Decarboxylative Borylation: New Avenues for the Preparation of Organoboron Compounds. Eur. J. Org. Chem. 2018, 2018, 3884–3890. [Google Scholar] [CrossRef]
- Mkhalid, I.A.I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C−H Activation for the Construction of C−B Bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef]
- Chow, W.K.; Yuen, O.Y.; Choy, P.Y.; So, C.M.; Lau, C.P.; Wong, W.T.; Kwong, F.Y. A decade advancement of transition metal-catalyzed borylation of aryl halides and sulfonates. RSC Adv. 2013, 3, 12518–12539. [Google Scholar] [CrossRef]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Murata, M.; Watanabe, S.; Masuda, Y. Novel Palladium(0)-Catalyzed Coupling Reaction of Dialkoxyborane with Aryl Halides: Convenient Synthetic Route to Arylboronates. J. Org. Chem. 1997, 62, 6458–6459. [Google Scholar] [CrossRef]
- Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. Palladium-Catalyzed Borylation of Aryl Halides or Triflates with Dialkoxyborane: A Novel and Facile Synthetic Route to Arylboronates. J. Org. Chem. 2000, 65, 164–168. [Google Scholar] [CrossRef]
- Lam, K.C.; Marder, T.B.; Lin, Z. Mechanism of the Palladium-Catalyzed Borylation of Aryl Halides with Pinacolborane. Organometallics 2010, 29, 1849–1857. [Google Scholar] [CrossRef]
- Iimura, S.; Wu, W. Palladium-catalyzed borylation of l-tyrosine triflate derivative with pinacolborane: Practical route to 4-borono-l-phenylalanine (l-BPA) derivatives. Tetrahedron Lett. 2010, 51, 1353–1355. [Google Scholar] [CrossRef]
- Gaich, T.; Baran, P.S. Aiming for the Ideal Synthesis. J. Org. Chem. 2010, 75, 4657–4673. [Google Scholar] [CrossRef]
- Lessing, T.; Müller, T.J.J. Sequentially Palladium-Catalyzed Processes in One-Pot Syntheses of Heterocycles. Appl. Sci. 2015, 5, 1803. [Google Scholar] [CrossRef]
- Baudoin, O.; Guénard, D.; Guéritte, F. Palladium-Catalyzed Borylation of Ortho-Substituted Phenyl Halides and Application to the One-Pot Synthesis of 2,2′-Disubstituted Biphenyls. J. Org. Chem. 2000, 65, 9268–9271. [Google Scholar] [CrossRef]
- Merkul, E.; Schäfer, E.; Müller, T.J.J. Rapid synthesis of bis(hetero)aryls by one-pot Masuda borylation-Suzuki coupling sequence and its application to concise total syntheses of meridianins A and G. Org. Biomol. Chem. 2011, 9, 3139–3141. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Buchwald, S.L. A Highly Active Catalyst for the Room-Temperature Amination and Suzuki Coupling of Aryl Chlorides. Angew. Chem. Int. Ed. 1999, 38, 2413–2416. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Singer, R.A.; Yang, B.H.; Buchwald, S.L. Highly Active Palladium Catalysts for Suzuki Coupling Reactions. J. Am. Chem. Soc. 1999, 121, 9550–9561. [Google Scholar] [CrossRef]
- Baudoin, O.; Claveau, F.; Thoret, S.; Herrbach, A.; Guénard, D.; Guéritte, F. Synthesis and biological evaluation of a-Ring biaryl-carbamate analogues of rhazinilam. Biorg. Med. Chem. 2002, 10, 3395–3400. [Google Scholar] [CrossRef]
- Baudoin, O.; Cesario, M.; Guénard, D.; Guéritte, F. Application of the Palladium-Catalyzed Borylation/Suzuki Coupling (BSC) Reaction to the Synthesis of Biologically Active Biaryl Lactams. J. Org. Chem. 2002, 67, 1199–1207. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Queiroz, M.-J.R.P.; Kirsch, G. Tandem palladium-catalyzed borylation and Suzuki coupling (BSC) to thienocarbazole precursors. Tetrahedron Lett. 2003, 44, 4327–4329. [Google Scholar] [CrossRef]
- Abreu, A.S.; Ferreira, P.M.T.; Queiroz, M.-J.R.P.; Ferreira, I.C.F.R.; Calhelha, R.C.; Estevinho, L.M. Synthesis of β-Benzo[b]thienyldehydrophenylalanine Derivatives by One-Pot Palladium-Catalyzed Borylation and Suzuki Coupling (BSC) and Metal-Assisted Intramolecular Cyclization-Studies of Fluorescence and Antimicrobial Activity. Eur. J. Org. Chem. 2005, 2005, 2951–2957. [Google Scholar] [CrossRef]
- Abreu, A.S.; Silva, N.O.; Ferreira, P.M.T.; Queiroz, M.-J.R.P. Palladium-catalyzed borylation and Suzuki coupling (BSC) to obtain β-substituted dehydroamino acid derivatives. Tetrahedron Lett. 2003, 44, 6007–6009. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Castanheira, E.M.S.; Lopes, T.C.T.; Cruz, Y.K.; Kirsch, G. Synthesis of fluorescent tetracyclic lactams by a “one pot” three steps palladium-catalyzed borylation, Suzuki coupling (BSC) and lactamization: DNA and polynucleotides binding studies. J. Photochem. Photobiol. A 2007, 190, 45–52. [Google Scholar] [CrossRef]
- Penhoat, M.; Levacher, V.; Dupas, G. Novel Extension of Meyers’ Methodology: Stereoselective Construction of Axially Chiral 7,5-Fused Bicyclic Lactams. J. Org. Chem. 2003, 68, 9517–9520. [Google Scholar] [CrossRef]
- Broutin, P.-E.; Čerňa, I.; Campaniello, M.; Leroux, F.; Colobert, F. Palladium-Catalyzed Borylation of Phenyl Bromides and Application in One-Pot Suzuki−Miyaura Biphenyl Synthesis. Org. Lett. 2004, 6, 4419–4422. [Google Scholar] [CrossRef]
- Duong, H.A.; Chua, S.; Huleatt, P.B.; Chai, C.L. Synthesis of biindolyls via palladium-catalyzed reactions. J. Org. Chem. 2008, 73, 9177–9180. [Google Scholar] [CrossRef]
- Krämer, C.S.; Zimmermann, T.J.; Sailer, M.; Müller, T.J.J. Syntheses of Phenothiazinylboronic Acid Derivatives—Suitable Starting Points for the Construction of Redox Active Materials. Synthesis 2002, 2002, 1163–1170. [Google Scholar] [CrossRef]
- Dorsch, D.; Sirrenberg, C.; Müller, T.J.J. Preparation of 4-(pyrrolopyridinyl)pyrimidinyl-2-amines as antitumor agents. PCT International Application WO2007107221A1, 27 September 2007. [Google Scholar]
- Dorsch, D.; Wuchrer, M.; Burgdorf, L.T.; Sirrenberg, C.; Esdar, C.; Mueller, T.J.J.; Merkul, E. 6-(Pyrrolopyridinyl)-pyrimidine-2-yl-amine derivatives and their use for the treatment of cancers and aids. PCT International Application WO2008155000A1, 24 December 2008. [Google Scholar]
- Dorsch, D.; Sirrenberg, C.; Müller, T.J.J.; Merkul, E. Preparation of 3-(4-pyridinyl)-1H-pyrrolo[2,3-b]pyridines as anti-tumor agents. Ger. Offen. DE102008025751A1, 3 December 2009. [Google Scholar]
- Dorsch, D.; Sirrenberg, C.; Müller, T.J.J.; Merkul, E.; Karapetyan, G. 7-Azaindole derivatives as kinase inhibitors and their preparation and use in the treatment of tumors. PCT International Application WO2012104007A2, 9 August 2012. [Google Scholar]
- Tasch, B.O.A.; Merkul, E.; Müller, T.J.J. One-Pot Synthesis of Diazine-Bridged Bisindoles and Concise Synthesis of the Marine Alkaloid Hyrtinadine A. Eur. J. Org. Chem. 2011, 2011, 4532–4535. [Google Scholar] [CrossRef]
- Kruppa, M.; Sommer, G.A.; Müller, T.J.J. Concise Syntheses of Marine (Bis)indole Alkaloids Meridianin C, D, F, and G and Scalaridine A via One-Pot Masuda Borylation-Suzuki Coupling Sequence. Molecules 2022, 27, 2233. [Google Scholar] [CrossRef] [PubMed]
- Tasch, B.O.A.; Antovic, D.; Merkul, E.; Müller, T.J.J. One-Pot Synthesis of Camalexins and 3,3′-Biindoles by the Masuda Borylation–Suzuki Arylation (MBSA) Sequence. Eur. J. Org. Chem. 2013, 2013, 4564–4569. [Google Scholar] [CrossRef]
- Drießen, D.; Stuhldreier, F.; Frank, A.; Stark, H.; Wesselborg, S.; Stork, B.; Müller, T.J.J. Novel meriolin derivatives as rapid apoptosis inducers. Bioorg. Med. Chem. 2019, 27, 3463–3468. [Google Scholar] [CrossRef]
- Rehberg, N.; Sommer, G.A.; Drießen, D.; Kruppa, M.; Adeniyi, E.T.; Chen, S.; Wang, L.; Wolf, K.; Tasch, B.O.A.; Ioerger, T.R.; et al. Nature-Inspired (di)Azine-Bridged Bisindole Alkaloids with Potent Antibacterial In Vitro and In Vivo Efficacy against Methicillin-Resistant Staphylococcus aureus. J. Med. Chem. 2020, 63, 12623–12641. [Google Scholar] [CrossRef]
- Zapf, A.; Ehrentraut, A.; Beller, M. A New Highly Efficient Catalyst System for the Coupling of Nonactivated and Deactivated Aryl Chlorides with Arylboronic Acids. Angew. Chem. Int. Ed. 2000, 39, 4153–4155. [Google Scholar] [CrossRef]
- Tasch, B.O.A.; Bensch, L.; Antovic, D.; Müller, T.J.J. Masuda borylation–Suzuki coupling (MBSC) sequence of vinylhalides and its application in a one-pot synthesis of 3,4-biarylpyrazoles. Org. Biomol. Chem. 2013, 11, 6113–6118. [Google Scholar] [CrossRef] [Green Version]
- Drießen, D.; Biesen, L.; Müller, T.J.J. Sequentially Catalyzed Three-Component Masuda–Suzuki–Sonogashira Synthesis of Fluorescent 2-Alkynyl-4-(7-azaindol-3-yl)pyrimidines: Three Palladium-Catalyzed Processes in a One-Pot Fashion. Synlett 2021, 32, 491–496. [Google Scholar] [CrossRef]
- Sommer, G.A.; Mataranga-Popa, L.N.; Czerwieniec, R.; Hofbeck, T.; Homeier, H.H.H.; Müller, T.J.J.; Yersin, H. Design of Conformationally Distorted Donor–Acceptor Dyads Showing Efficient Thermally Activated Delayed Fluorescence. J. Phys. Chem. Lett. 2018, 9, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Biesen, L.; Müller, T.J.J. Solid-state emissive biphenylene bridged bisaroyl-S,N-ketene acetals as distinct aggregation-induced enhanced emitters and fluorometric probes. Aggregate 2021, 2, e105. [Google Scholar] [CrossRef]




























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruppa, M.; Müller, T.J.J. Masuda Borylation–Suzuki Coupling (MBSC) Sequence: A One-Pot Process to Access Complex (hetero)Biaryls. Catalysts 2023, 13, 350. https://doi.org/10.3390/catal13020350
Kruppa M, Müller TJJ. Masuda Borylation–Suzuki Coupling (MBSC) Sequence: A One-Pot Process to Access Complex (hetero)Biaryls. Catalysts. 2023; 13(2):350. https://doi.org/10.3390/catal13020350
Chicago/Turabian StyleKruppa, Marco, and Thomas J. J. Müller. 2023. "Masuda Borylation–Suzuki Coupling (MBSC) Sequence: A One-Pot Process to Access Complex (hetero)Biaryls" Catalysts 13, no. 2: 350. https://doi.org/10.3390/catal13020350
APA StyleKruppa, M., & Müller, T. J. J. (2023). Masuda Borylation–Suzuki Coupling (MBSC) Sequence: A One-Pot Process to Access Complex (hetero)Biaryls. Catalysts, 13(2), 350. https://doi.org/10.3390/catal13020350