Alkali and Alkaline Earth Metals (K, Ca, Sr) Promoted Cu/SiO2 Catalyst for Hydrogenation of Methyl Acetate to Ethanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of the Catalysts
2.2. Crystalline Phase and Morphology
2.3. H2-TPR and H2-TPD
2.4. Chemical States of Surface Species
3. Experimental
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalyst Tests
3.5. Catalytic Performance and Stability
3.5.1. Effect of Reaction Temperature
3.5.2. Effect of Liquid Hourly Space Velocity
3.5.3. Effect of Pressure
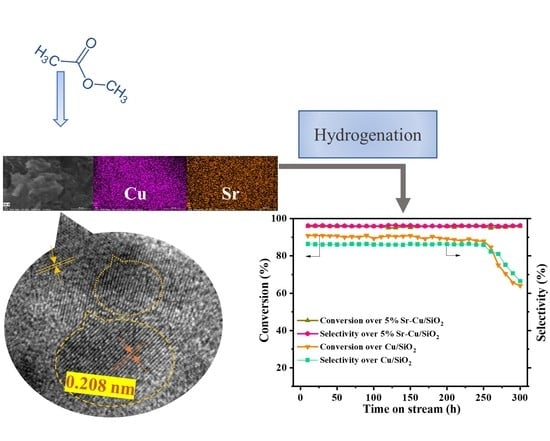
3.5.4. Catalytic Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roehr, M. The Biotechnology of Ethanol: Classical and Future Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; ISBN 9783527602346. [Google Scholar]
- Jiao, J.; Li, J.; Bai, Y. Ethanol as a Vehicle Fuel in China: A Review from the Perspectives of Raw Material Resource, Vehicle, and Infrastructure. J. Clean. Prod. 2018, 180, 832–845. [Google Scholar] [CrossRef]
- Dueso, C.; Ortiz, M.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. Reduction and Oxidation Kinetics of Nickel-Based Oxygen-Carriers for Chemical-Looping Combustion and Chemical-Looping Reforming. Chem. Eng. J. 2012, 188, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; San, X.; Zhang, Y.; Ichii, T.; Meng, M.; Tan, Y.; Tsubaki, N. Direct Synthesis of Ethanol from Dimethyl Ether and Syngas over Combined H-Mordenite and Cu/ZnO Catalysts. ChemSusChem 2010, 3, 1192–1199. [Google Scholar] [CrossRef]
- Wang, S.; Guo, W.; Wang, H.; Zhu, L.; Yin, S.; Qiu, K. Effect of the Cu/SBA-15 Catalyst Preparation Method on Methyl Acetate Hydrogenation for Ethanol Production. N. J. Chem. 2014, 38, 2792. [Google Scholar] [CrossRef]
- Ye, C.; Guo, C.; Sun, C.; Zhang, Y. Effect of Mn Doping on the Activity and Stability of Cu–SiO2 Catalysts for the Hydrogenation of Methyl Acetate to Ethanol. RSC Adv. 2016, 6, 113796–113802. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Kang, H.; Chen, J. Highly Dispersed Silica-Supported Copper Nanoparticles Prepared by Precipitation−Gel Method: A Simple but Efficient and Stable Catalyst for Glycerol Hydrogenolysis. Chem. Mater. 2008, 20, 5090–5099. [Google Scholar] [CrossRef]
- Ye, C.-L.; Guo, C.-L.; Zhang, J.-L. Highly Active and Stable CeO2–SiO2 Supported Cu Catalysts for the Hydrogenation of Methyl Acetate to Ethanol. Fuel Process. Technol. 2016, 143, 219–224. [Google Scholar] [CrossRef]
- Huang, X.; Ma, M.; Miao, S.; Zheng, Y.; Chen, M.; Shen, W. Hydrogenation of Methyl Acetate to Ethanol over a Highly Stable Cu/SiO2 Catalyst: Reaction Mechanism and Structural Evolution. Appl. Catal. A Gen. 2017, 531, 79–88. [Google Scholar] [CrossRef]
- Chen, L.-F.; Guo, P.-J.; Qiao, M.-H.; Yan, S.-R.; Li, H.-X.; Shen, W.; Xu, H.-L.; Fan, K.-N. Cu/SiO2 Catalysts Prepared by the Ammonia-Evaporation Method: Texture, Structure, and Catalytic Performance in Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Fan, K.; Dai, W.-L. Ion-Exchange Temperature Effect on Cu/HMS Catalysts for the Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. ChemCatChem 2010, 2, 206–213. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.L.; Li, H.; Fan, K. Highly Active and Selective Copper-Containing HMS Catalyst in the Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Appl. Catal. A Gen. 2008, 349, 91–99. [Google Scholar] [CrossRef]
- Di, W.; Cheng, J.; Tian, S.; Li, J.; Chen, J.; Sun, Q. Synthesis and Characterization of Supported Copper Phyllosilicate Catalysts for Acetic Ester Hydrogenation to Ethanol. Appl. Catal. A Gen. 2016, 510, 244–259. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of Boric Oxide Doping on the Stability and Activity of a Cu-SiO2 catalyst for Vapor-Phase Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, X. The Influence of Different Precipitants on the Copper-Based Catalysts for Hydrogenation of Ethyl Acetate to Ethanol. Int. J. Hydrogen Energy 2014, 39, 10951–10958. [Google Scholar] [CrossRef]
- Yang, D.; Sararuk, C.; Suzuki, K.; Li, Z.; Li, C. Effect of Calcination Temperature on the Catalytic Activity of VPO for Aldol Condensation of Acetic Acid and Formalin. Chem. Eng. J. 2016, 300, 160–168. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, Q.; Qin, Z.; Li, Q.; Feng, X.; Song, Z.; Cai, Z.; Liu, Y.; Chen, X.; Chen, D.; et al. Reversing Titanium Oligomer Formation towards High-Efficiency and Green Synthesis of Titanium-Containing Molecular Sieves. Angew. Chem. Int. Ed. 2021, 60, 3443–3448. [Google Scholar] [CrossRef]
- Runeberg, J.; Baiker, A.; Kijenski, J. Copper Catalyzed Amination of Ethylene Glycol. Appl. Catal. 1985, 17, 309–319. [Google Scholar] [CrossRef]
- Montassier, C.; Giraud, D.; Barbier, J. Polyol Conversion by Liquid Phase Heterogeneous Catalysis over Metals. Prep. Catal. V-Sci. Bases Prep. Heterog. Catal. Proc. Fifth Int. Symp. 1988, 41, 165–170. [Google Scholar]
- Brands, D.S.; Poels, E.K.; Bliek, A. Ester Hydrogenolysis over Promoted Cu/SiO2 Catalysts. Appl. Catal. A Gen. 1999, 184, 279–289. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, J.; Zhang, J.; Wang, S.; Zhao, Y.; Ma, X. Hydrogenation of Methyl Acetate to Ethanol by Cu/ZnO Catalyst Encapsulated in SBA-15. AIChE J. 2017, 63, 2839–2849. [Google Scholar] [CrossRef]
- Ren, Z.; Younis, M.N.; Zhao, H.; Li, C.; Yang, X.; Wang, E.; Wang, G. Silver Modified Cu/SiO2 Catalyst for the Hydrogenation of Methyl Acetate to Ethanol. Chin. J. Chem. Eng. 2020, 28, 1612–1622. [Google Scholar] [CrossRef]
- Yan, H.; Li, S.; Feng, X.; Lu, J.; Zheng, X.; Li, R.; Zhou, X.; Chen, X.; Liu, Y.; Chen, D.; et al. Rational Screening of Metal Catalysts for Selective Oxidation of Glycerol to Glyceric Acid from Microkinetic Analysis. AIChE J. 2023, 69, e17868. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, M.; Feng, X.; Zhao, S.; Zhou, X.; Li, S.; Zha, M.; Meng, F.; Chen, X.; Liu, Y.; et al. PO43− Coordinated Robust Single-Atom Platinum Catalyst for Selective Polyol Oxidation. Angew. Chem. Int. Ed. 2022, 61, e202116059. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Zhao, Y.; Lv, J.; Wang, S.; Ma, X. Insight into the Balancing Effect of Active Cu Species for Hydrogenation of Carbon–Oxygen Bonds. ACS Catal. 2015, 5, 6200–6208. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, H.; Cui, F.; Zuo, J.; Chen, J.; Xia, C. Effects of the Precipitation Agents and Rare Earth Additives on the Structure and Catalytic Performance in Glycerol Hydrogenolysis of Cu/SiO2 catalysts Prepared by Precipitation-Gel Method. Catal. Today 2014, 234, 223–232. [Google Scholar] [CrossRef]
- Qin, H.; Guo, C.; Sun, C.; Zhang, J. Influence of the Support Composition on the Hydrogenation of Methyl Acetate over Cu/MgO-SiO2 Catalysts. J. Mol. Catal. A: Chem. 2015, 409, 79–84. [Google Scholar] [CrossRef]
- Zhao, Y.; Shan, B.; Wang, Y.; Zhou, J.; Wang, S.; Ma, X. An Effective CuZn-SiO2 Bimetallic Catalyst Prepared by Hydrolysis Precipitation Method for the Hydrogenation of Methyl Acetate to Ethanol. Ind. Eng. Chem. Res. 2018, 57, 4526–4534. [Google Scholar] [CrossRef]
- Ren, Z.; Younis, M.N.; Li, C.; Li, Z.; Yang, X.; Wang, G. Highly Active Ce, Y, La-Modified Cu/SiO2 Catalysts for Hydrogenation of Methyl Acetate to Ethanol. RSC Adv. 2020, 10, 5590–5603. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Younis, M.N.; Wu, H.; Li, C.; Yang, X.; Wang, G. Design and Synthesis of La-Modified Copper Phyllosilicate Nanotubes for Hydrogenation of Methyl Acetate to Ethanol. Catal. Lett. 2021, 151, 3089–3102. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, L.; Guo, X.; Mao, J.; Zhang, S. Selective Hydrogenolysis of Glycerol to Propanediols on Supported Cu-Containing Bimetallic Catalysts. Green Chem. 2010, 12, 1835–1843. [Google Scholar] [CrossRef]
- Yaseen, M.; Shakirullah, M.; Ahmad, I.; Rahman, A.U.; Rahman, F.U.; Usman, M.; Razzaq, R. Simultaneous Operation of Dibenzothiophene Hydrodesulfurization and Methanol Reforming Reactions over Pd Promoted Alumina Based Catalysts. J. Fuel Chem. Technol. 2012, 40, 714–720. [Google Scholar] [CrossRef]
- Muhammad, Y.; Rashid, H.U.; Subhan, S.; Rahman, A.U.; Sahibzada, M.; Tong, Z. Boosting the Hydrodesulfurization of Dibenzothiophene Efficiency of Mn Decorated (Co/Ni)-Mo/Al2O3 Catalysts at Mild Temperature and Pressure by Coupling with Phosphonium Based Ionic Liquids. Chem. Eng. J. 2019, 375, 121957. [Google Scholar] [CrossRef]
- Muhammad, Y.; Rahman, A.U.; Rashid, H.U.; Sahibzada, M.; Subhan, S.; Tong, Z. Hydrodesulfurization of Dibenzothiophene Using Pd-Promoted Co–Mo/Al2O3 and Ni–Mo/Al2O3 Catalysts Coupled with Ionic Liquids at Ambient Operating Conditions. RSC Adv. 2019, 9, 10371–10385. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, Z.; Kang, H.; Li, X.; Xia, C.; Chen, J.; Liu, H. Efficient Bimetallic NiCu-SiO2 Catalysts for Selective Hydrogenolysis of Xylitol to Ethylene Glycol and Propylene Glycol. Appl. Catal. B: Environ. 2018, 220, 251–263. [Google Scholar] [CrossRef]
- Yan, H.; Qin, H.; Feng, X.; Jin, X.; Liang, W.; Sheng, N.; Zhu, C.; Wang, H.; Yin, B.; Liu, Y.; et al. Synergistic Pt/MgO/SBA-15 Nanocatalysts for Glycerol Oxidation in Base-Free Medium: Catalyst Design and Mechanistic Study. J. Catal. 2019, 370, 434–446. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Shi, L. Zn Promoted Cu–Al Catalyst for Hydrogenation of Ethyl Acetate to Alcohol. J. Ind. Eng. Chem. 2014, 20, 2341–2347. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, H.; Zheng, J.; Duan, X.; Yuan, Y. Lanthanum Oxide-Modified Cu/SiO2 as a High-Performance Catalyst for Chemoselective Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. ACS Catal. 2013, 3, 2738–2749. [Google Scholar] [CrossRef]
- Ying, J.; Han, X.; Ma, L.; Lu, C.; Feng, F.; Zhang, Q.; Li, X. Effects of Basic Promoters on the Catalytic Performance of Cu/SiO2 in the Hydrogenation of Dimethyl Maleate. Catalysts 2019, 9, 704. [Google Scholar] [CrossRef] [Green Version]
- Gluhoi, A.C.; Nieuwenhuys, B.E. Structural and Chemical Promoter Effects of Alkali (Earth) and Cerium Oxides in CO Oxidation on Supported Gold. Catal. Today 2007, 122, 226–232. [Google Scholar] [CrossRef]
- Gluhoi, A.C.; Bogdanchikova, N.; Nieuwenhuys, B.E. Alkali (Earth)-Doped Au/Al2O3 Catalysts for the Total Oxidation of Propene. J. Catal. 2005, 232, 96–101. [Google Scholar] [CrossRef]
- Yang, D.; Li, J.; Wen, M.; Song, C. Enhanced Activity of Ca-Doped Cu/ZrO2 for Nitrogen Oxides Reduction with Propylene in the Presence of Excess Oxygen. Catal. Today 2008, 139, 2–7. [Google Scholar] [CrossRef]
- Pellegrini, R.; Leofanti, G.; Agostini, G.; Bertinetti, L.; Bertarione, S.; Groppo, E.; Zecchina, A.; Lamberti, C. Influence of K-Doping on a Pd/SiO2-Al2O3 Catalyst. J. Catal. 2009, 267, 40–49. [Google Scholar] [CrossRef]
- Evans, J.W.; Wainwright, M.S.; Bridgewater, A.J.; Young, D.J. On the Determination of Copper Surface Area by Reaction with Nitrous Oxide. Appl. Catal. 1983, 7, 75–83. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Ding, G.; Zheng, H.; Li, Y. Modification of the Supported Cu/SiO2 Catalyst by Alkaline Earth Metals in the Selective Conversion of 1,4-Butanediol to γ-Butyrolactone. Appl. Catal. A Gen. 2012, 443–444, 191–201. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Lu, G. Studies on the Active Species and on Dispersion of Cu in Cu/SiO2 and Cu/Zn/SiO2 for Hydrogen Production via Methanol Partial Oxidation. Int. J. Hydrogen Energy 2003, 28, 151–158. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.-F.; Louis, C. Conditions of Formation of Copper Phyllosilicates in Silica-Supported Copper Catalysts Prepared by Selective Adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
- To, D.T.; Lin, Y.C. Copper Phyllosilicates-Derived Catalysts in the Production of Alcohols from Hydrogenation of Carboxylates, Carboxylic Acids, Carbonates, Formyls, and CO2: A Review. Catalysts 2021, 11, 255. [Google Scholar] [CrossRef]
- Maul, J.; Brito, A.S.; de Oliveira, A.L.M.; Lima, S.J.G.; Maurera, M.A.M.A.; Keyson, D.; Souza, A.G.; Santos, I.M.G. Influence of the Synthesis Media in the Properties of CuO Obtained by Microwave-Assisted Hydrothermal Method. J. Therm. Anal. Calorim. 2011, 106, 519–523. [Google Scholar] [CrossRef]
- Lu, L.; Huang, X. Room Temperature Electrochemical Synthesis of CuO Flower-like Microspheres and Their Electrooxidative Activity towards Hydrogen Peroxide. Microchim. Acta 2011, 175, 151. [Google Scholar] [CrossRef]
- Hou, X.; Zhao, J.; Liu, J.; Han, Y.; Pei, Y.; Ren, J. Activated Carbon Aerogel Supported Copper Catalysts for the Hydrogenation of Methyl Acetate to Ethanol: Effect of KOH Activation. N. J. Chem. 2019, 43, 9430–9438. [Google Scholar] [CrossRef]
- Wilmer, H.; Genger, T.; Hinrichsen, O. The Interaction of Hydrogen with Alumina-Supported Copper Catalysts: A Temperature-Programmed Adsorption/Temperature-Programmed Desorption/Isotopic Exchange Reaction Study. J. Catal. 2003, 215, 188–198. [Google Scholar] [CrossRef]
- Scholten, J.J.F.; Pijpers, A.P.; Hustings, A.M.L. Surface Characterization of Supported and Nonsupported Hydrogenation Catalysts. Catal. Rev. 1985, 27, 151–206. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, H.-B.; Lin, G.-D.; Yuan, Y.-Z.; Tsai, K.R. Highly Active CNT-Promoted Cu-ZnO-Al2O3 Catalyst for Methanol Synthesis from H2/CO/CO2. Catal. Lett. 2003, 85, 237–246. [Google Scholar] [CrossRef]
- Tu, Y.J.; Chen, Y.W. Effects of Alkali Metal Oxide Additives on Cu/SiO2 Catalyst in the Dehydrogenation of Ethanol. Ind. Eng. Chem. Res. 2001, 40, 5889–5893. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of Ethanol via Syngas on Cu/SiO2 Catalysts with Balanced Cu0–Cu+ Sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.-L.; Fan, K. The Nature of Active Copper Species in Cu-HMS Catalyst for Hydrogenation of Dimethyl Oxalate to Ethylene Glycol: New Insights on the Synergetic Effect between Cu0 and Cu+. J. Phys. Chem. C 2009, 113, 11003–11013. [Google Scholar] [CrossRef]
- Shen, J.; Rao, C.; Fu, Z.; Feng, X.; Liu, J.; Fan, X.; Peng, H.; Xu, X.; Tan, C.; Wang, X. The Influence on the Structural and Redox Property of CuO by Using Different Precursors and Precipitants for Catalytic Soot Combustion. Appl. Surf. Sci. 2018, 453, 204–213. [Google Scholar] [CrossRef]
- Wen, C.; Cui, Y.; Dai, W.L.; Xie, S.; Fan, K. Solvent Feedstock Effect: The Insights into the Deactivation Mechanism of Cu/SiO2 Catalysts for Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Chem. Commun. 2013, 49, 5195–5197. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, X.; Cui, Y.; Zhang, H.; Liao, D. Effect of Feedstock Solvent on the Stability of Cu/SiO2 Catalyst for Vapor-Phase Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Chem. Commun. 2012, 48, 1177–1179. [Google Scholar] [CrossRef]
- Van Der Grift, C.J.G.; Wielers, A.F.H.; Jogh, B.P.J.; Van Beunum, J.; De Boer, M.; Versluijs-Helder, M.; Geus, J.W. Effect of the Reduction Treatment on the Structure and Reactivity of Silica-Supported Copper Particles. J. Catal. 1991, 131, 178–189. [Google Scholar] [CrossRef]












| Catalyst | Content a | SBET b (m2/g) | BJHVp b (cm3/g) | BJHDp b (nm) | SCu0 c (m2/g) | SCu+ d(m2/g) | SCu (m2/g) | |
|---|---|---|---|---|---|---|---|---|
| Cu | M | |||||||
| 30%Cu/SiO2 | 30.2 | - | 50.0 | 0.19 | 16.8 | 33.7 | 31.2 | 64.9 |
| 5%K-30%Cu/SiO2 | 26.6 | 4.6 | 50.2 | 0.20 | 19.0 | 34.9 | 37.8 | 72.7 |
| 5%Ca-30%Cu/SiO2 | 27.1 | 4.4 | 114.8 | 0.73 | 23.9 | 35.4 | 34.2 | 69.6 |
| 1%Sr-30%Cu/SiO2 | 28.3 | 0.92 | 79.9 | 0.52 | 23.4 | 36.1 | 38.6 | 74.7 |
| 5%Sr-30%Cu/SiO2 | 26.8 | 4.5 | 119.3 | 0.76 | 26.8 | 36.2 | 44.6 | 80.8 |
| 10%Sr-30%Cu/SiO2 | 25.9 | 8.7 | 41.4 | 0.25 | 21.5 | 34.5 | 38.6 | 73.1 |
| AS-40 SiO2 | -- | -- | 129–155 | 20–24 | ||||
| Catalyst | 2θ of Cu (111) | dCu (nm) | dCu2+ (nm) |
|---|---|---|---|
| 30%Cu/SiO2 | 43.4 | 4.8 | 4.4 |
| 5%K-30% Cu/SiO2 | 43.4 | 9.5 | 4.3 |
| 5%Ca-30% Cu/SiO2 | 43.2 | 4.6 | 5.3 |
| 1%Sr-30% Cu/SiO2 | 43.1 | 3.7 | 4.0 |
| 5%Sr-30% Cu/SiO2 | 43.0 | 4.0 | 4.8 |
| 10%Sr-30% Cu/SiO2 | 43.2 | 4.9 | 6.2 |
| Catalyst | KE (eV) | AP (eV) | Cu 2p3/2 BE (eV) | XCu+ | ||
|---|---|---|---|---|---|---|
| Cu+ | Cu0 | Cu+ | Cu0 | |||
| 30%Cu/SiO2 | 913.7 | 917.5 | 1847.4 | 1851.3 | 932.3 | 0.48 |
| 5%K-30%Cu/SiO2 | 913.7 | 917.5 | 1847.1 | 1851.0 | 932.1 | 0.43 |
| 5%Ca-30%Cu/SiO2 | 913.6 | 917.5 | 1847.3 | 1851.3 | 932.0 | 0.42 |
| 1%Sr-30%Cu/SiO2 | 913.6 | 917.6 | 1847.5 | 1851.4 | 932.2 | 0.50 |
| 5%Sr-30%Cu/SiO2 | 913.7 | 917.5 | 1847.5 | 1851.3 | 932.2 | 0.60 |
| 10%Sr-30%Cu/SiO2 | 913.6 | 917.6 | 1847.5 | 1851.4 | 932.2 | 0.54 |
| Catalyst | MA Conversion/ % a | Ethanol Selectivity/ % a | Yield/ % a | STY/ g Ethanol/(g cat h) a |
|---|---|---|---|---|
| 30%Cu/SiO2 | 90.8 | 86.3 | 78.4 | 0.91 |
| 5%K-30%Cu/SiO2 | 92.2 | 91.3 | 84.2 | 0.97 |
| 5%Ca-30%Cu/SiO2 | 91.1 | 90.2 | 82.2 | 0.95 |
| 1%Sr-30%Cu/SiO2 | 91.8 | 91.2 | 83.7 | 0.97 |
| 5%Sr-30%Cu/SiO2 | 95.8 | 96.2 | 92.2 | 1.07 |
| 10%Sr-30%Cu/SiO2 | 87.7 | 88.0 | 77.3 | 0.89 |
| 5%Sr/SiO2 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younis, M.N.; Ren, Z.; Li, C.; Wang, E.; Li, J. Alkali and Alkaline Earth Metals (K, Ca, Sr) Promoted Cu/SiO2 Catalyst for Hydrogenation of Methyl Acetate to Ethanol. Catalysts 2023, 13, 450. https://doi.org/10.3390/catal13020450
Younis MN, Ren Z, Li C, Wang E, Li J. Alkali and Alkaline Earth Metals (K, Ca, Sr) Promoted Cu/SiO2 Catalyst for Hydrogenation of Methyl Acetate to Ethanol. Catalysts. 2023; 13(2):450. https://doi.org/10.3390/catal13020450
Chicago/Turabian StyleYounis, Muhammad Naeem, Zhiheng Ren, Chunshan Li, Erqiang Wang, and Jie Li. 2023. "Alkali and Alkaline Earth Metals (K, Ca, Sr) Promoted Cu/SiO2 Catalyst for Hydrogenation of Methyl Acetate to Ethanol" Catalysts 13, no. 2: 450. https://doi.org/10.3390/catal13020450
APA StyleYounis, M. N., Ren, Z., Li, C., Wang, E., & Li, J. (2023). Alkali and Alkaline Earth Metals (K, Ca, Sr) Promoted Cu/SiO2 Catalyst for Hydrogenation of Methyl Acetate to Ethanol. Catalysts, 13(2), 450. https://doi.org/10.3390/catal13020450