Photocatalytic Evaluation of the Ternary Composite CdSO4-ZnAl LDH/ZnS in Hydrogen Production without a Sacrificial Reagent
Abstract
:1. Introduction
2. Results
2.1. X-ray Diffraction
2.2. Diffuse Reflectance Spectroscopy (DRS)
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Photoluminescence
2.5. N2 Physisorption
2.6. Rietveld Analysis and Energy Dispersive Spectrometry (EDS)
2.7. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
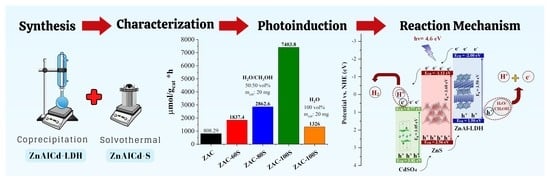
2.8. Photocatalysis Assessment
2.9. Reaction Mechanism
2.9.1. Charge Carriers’ Formation
2.9.2. Water Splitting
2.9.3. Photocatalytic Reforming
3. Materials and Methods
3.1. Synthesis of the LDH Materials
3.2. ZAC Sulfuration
3.3. Characterization of the Powder Photocatalysts
3.4. Photocatalysts Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farias, C.B.B.; Barreiros, R.C.S.; da Silva, M.F.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies 2022, 15, 311. [Google Scholar] [CrossRef]
- Kundu, B.K.; Han, G.; Sun, Y. Derivatized Benzothiazoles as Two-Photon-Absorbing Organic Photosensitizers Active under Near Infrared Light Irradiation. J. Am. Chem. Soc. 2023, 145, 3535–3542. [Google Scholar] [CrossRef]
- Tzompantzi-Flores, C.; Castillo-Rodríguez, J.C.; Gómez, R.; Hernández, R.P.; Santolalla-Vargas, C.E.; Tzompantzi, F. Photocatalytic Evaluation of the ZrO2:Zn5(OH)6(CO3)2 Composite for the H2 Production via Water Splitting. Top. Catal. 2020, 63, 575–585. [Google Scholar] [CrossRef]
- Chubar, N.; Gilmour, R.; Gerda, V.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered double hydroxides as the next generation inorganic anion exchangers: Synthetic methods versus applicability. Adv. Colloid Interface Sci. 2017, 245, 62–80. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, S.; Pal, B. A brief review on modified layered double hydroxides for H2 production through photoinduced H2O splitting. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100451. [Google Scholar] [CrossRef]
- Li, A.; Deng, H.; Ye, C.; Jiang, Y. Fabrication and characterization of novel ZnAl-layered double hydroxide for the superadsorption of organic contaminants from wastewater. ACS Omega 2020, 5, 15152–15161. [Google Scholar] [CrossRef]
- Suárez-Quezada, M.; Romero-Ortiz, G.; Samaniego-Benítez, J.; Suárez, V.; Mantilla, A. H2 production by the water splitting reaction using photocatalysts derived from calcined ZnAl LDH. Fuel 2018, 240, 262–269. [Google Scholar] [CrossRef]
- He, X.; Qiu, X.; Chen, J. Preparation of Fe(II)–Al layered double hydroxides: Application to the adsorption/reduction of chromium. Colloids Surf. A Physicochem. Eng. Asp. 2016, 516, 362–374. [Google Scholar] [CrossRef]
- Rodriguez-Rivas, F.; Pastor, A.; de Miguel, G.; Cruz-Yusta, M.; Pavlovic, I.; Sánchez, L. Cr3+ substituted Zn-Al layered double hydroxides as UV–Vis light photocatalysts for NO gas removal from the urban environment. Sci. Total Environ. 2019, 706, 136009. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, B.; Hu, Z.; Zhen, M.; Guo, S.-Q.; Dong, F. Uncovering the synergy between Mn substitution and O vacancy in ZnAl-LDH photocatalyst for efficient toluene removal. Appl. Catal. B Environ. 2021, 296, 120376. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Matsui, Y.; Shirahata, N.; Dumait, N.; Cordier, S.; Grasset, F.; Ohashi, N.; Uchikoshi, T. Zn-Al layered double hydroxide-based nanocomposite functionalized with an octahedral molybdenum cluster exhibiting prominent photoactive and oxidation properties. Appl. Clay Sci. 2020, 196, 105765. [Google Scholar] [CrossRef]
- Gil, J.J.; Aguilar-Martínez, O.; Piña-Pérez, Y.; Pérez-Hernández, R.; Santolalla-Vargas, C.; Gómez, R.; Tzompantzi, F. Efficient ZnS–ZnO/ZnAl-LDH composite for H2 production by photocatalysis. Renew. Energy 2019, 145, 124–132. [Google Scholar] [CrossRef]
- Thite, V.D.; Giripunje, S.M. Adsorption and photocatalytic performance of ZnAl layered double hydroxide nanoparticles in removal of methyl orange dye. Nanotechnol. Environ. Eng. 2022, 7, 57–66. [Google Scholar] [CrossRef]
- Mendoza-Damián, G.; Tzompantzi, F.; Mantilla, A.; Pérez-Hernández, R.; Hernández-Gordillo, A. Improved photocatalytic activity of SnO2–ZnAl LDH prepared by one step Sn4+ incorporation. Appl. Clay Sci. 2016, 121–122, 127–136. [Google Scholar] [CrossRef]
- Lalithadevi, B.; Rao, K.M.; Ramananda, D. Investigations on structural and optical properties of starch capped ZnS nanoparticles synthesized by microwave irradiation method. Chem. Phys. Lett. 2018, 700, 74–79. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Tzompantzi-Flores, C.; Portillo-Vélez, N.; Castillo-Rodríguez, J.; Gómez, R.; Hernández, R.P.; Santolalla-Vargas, C. Preparation and characterization of the polycrystalline material Zn5(OH)6(CO3)2. Determination of the active species in oxide-reduction processes. Fuel 2020, 281, 118471. [Google Scholar] [CrossRef]
- Torres-Ricárdez, R.; Lizama-Tzec, F.; García-Mendoza, M.; Ramírez-Morales, E.; Rojas-Blanco, L.; Ramírez-Betancour, R.; Martínez-Solís, F.; Pérez-Hernández, G. Electrodeposited stoichiometric zinc sulfide films. Ceram. Int. 2020, 46, 10490–10494. [Google Scholar] [CrossRef]
- Kundu, B.K.; Das, M.; Ganguly, R.; Bhobe, P.A.; Mukhopadhyay, S. Role of zeolite encapsulated Cu(II) complexes in electron transfer as well as peroxy radical intermediates formation during oxidation of thioanisole. J. Catal. 2020, 389, 305–316. [Google Scholar] [CrossRef]
- Kundu, B.K.; Chhabra, V.; Malviya, N.; Ganguly, R.; Mishra, G.S.; Mukhopadhyay, S. Zeolite encapsulated host-guest Cu(II) Schiff base complexes: Superior activity towards oxidation reactions over homogenous catalytic systems. Microporous Mesoporous Mater. 2018, 271, 100–117. [Google Scholar] [CrossRef]
- Berlin, I.J.; Anitha, V.S.; Thomas, P.V.; Joy, K. Influence of oxygen atmosphere on the photoluminescence properties of sol–gel derived ZrO2 thin films. J. Sol-Gel Sci. Technol. 2012, 64, 289–296. [Google Scholar] [CrossRef]
- Saiah, F.B.D.; Su, B.-L.; Bettahar, N. Nickel–iron layered double hydroxide (LDH): Textural properties upon hydrothermal treatments and application on dye sorption. J. Hazard. Mater. 2009, 165, 206–217. [Google Scholar] [CrossRef]
- Bian, X.; Zhang, S.; Zhao, Y.; Shi, R.; Zhang, T. Layered double hydroxide-based photocatalytic materials toward renewable solar fuels production. Infomat 2021, 3, 719–738. [Google Scholar] [CrossRef]
- Wu, A.; Jing, L.; Wang, J.; Qu, Y.; Xie, Y.; Jiang, B.; Tian, C.; Fu, H. ZnO-dotted porous ZnS cluster microspheres for high efficient, Pt-free photocatalytic hydrogen evolution. Sci. Rep. 2015, 5, srep08858. [Google Scholar] [CrossRef] [Green Version]
- Materials Data on CdSO4, Materials Project, United States. 2020. Available online: https://materialsproject.org/materials/mp-5679 (accessed on 20 December 2022).
- Spagnolo, G.S.; Postiglione, A.; De Angelis, I. Simple equipment for teaching internal photoelectric effect. Phys. Educ. 2020, 55, 055011. [Google Scholar] [CrossRef]
- Hussein, E.M.A. (Ed.) Chapter one-mechanisms. In Radiation Mechanics; Elsevier Science Ltd.: Oxford, UK, 2007; pp. 1–65. [Google Scholar] [CrossRef]
- Tan, X.; Ng, S.; Mohamed, A.R.; Ong, W. Point-to-face contact heterojunctions: Interfacial design of 0D nanomaterials on 2D g-C3N4 towards photocatalytic energy applications. Carbon Energy 2022, 4, 665–730. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Guzman, F.; Chuang, S.S.C.; Yang, C. Role of Methanol Sacrificing Reagent in the Photocatalytic Evolution of Hydrogen. Ind. Eng. Chem. Res. 2013, 52, 61–65. [Google Scholar] [CrossRef]











| Material | Lattice Parameters (Å) | Crystal Size (nm) | ||
|---|---|---|---|---|
| d(003) | a = 2 × d(110) | c = 3 × d(003) | ||
| ZAC | 7.55 | 3.06 | 22.65 | 27.58 |
| ZAC-60S | 7.55 | 3.06 | 22.65 | 31.59 |
| ZAC-80S | 7.56 | 3.06 | 22.68 | 25.47 |
| ZAC-100S | 7.50 | --- | 22.52 | 11.39 |
| Material | d(111) | a = (4 × r/20.5) | a = b = c | Crystal size (nm) |
| ZAC-100S | 3.1 | 5.6 | 5.6 | 37.8 |
| Material | Eg (eV) | Crystal Size (nm) | SBET (m2 g−1) | Vpore (m3 g−1) |
|---|---|---|---|---|
| ZAC | 5.66 | 27.58 | 18 | 0.12 |
| ZAC-60S | 3.57 | 31.59 | 40 | 0.16 |
| ZAC-80S | 3.58 | 25.47 | 43 | 0.16 |
| ZAC-100S | 3.67 | 11.37(003) 37.8(111) | 105 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Villegas, A.G.; Tzompantzi-Flores, C.; Hernández, R.P.; Barrera-Rodríguez, A.; Tzompantzi, F.; Gómez, R. Photocatalytic Evaluation of the Ternary Composite CdSO4-ZnAl LDH/ZnS in Hydrogen Production without a Sacrificial Reagent. Catalysts 2023, 13, 593. https://doi.org/10.3390/catal13030593
Romero-Villegas AG, Tzompantzi-Flores C, Hernández RP, Barrera-Rodríguez A, Tzompantzi F, Gómez R. Photocatalytic Evaluation of the Ternary Composite CdSO4-ZnAl LDH/ZnS in Hydrogen Production without a Sacrificial Reagent. Catalysts. 2023; 13(3):593. https://doi.org/10.3390/catal13030593
Chicago/Turabian StyleRomero-Villegas, Angela G., Clara Tzompantzi-Flores, Raúl Pérez Hernández, Arturo Barrera-Rodríguez, Francisco Tzompantzi, and Ricardo Gómez. 2023. "Photocatalytic Evaluation of the Ternary Composite CdSO4-ZnAl LDH/ZnS in Hydrogen Production without a Sacrificial Reagent" Catalysts 13, no. 3: 593. https://doi.org/10.3390/catal13030593
APA StyleRomero-Villegas, A. G., Tzompantzi-Flores, C., Hernández, R. P., Barrera-Rodríguez, A., Tzompantzi, F., & Gómez, R. (2023). Photocatalytic Evaluation of the Ternary Composite CdSO4-ZnAl LDH/ZnS in Hydrogen Production without a Sacrificial Reagent. Catalysts, 13(3), 593. https://doi.org/10.3390/catal13030593