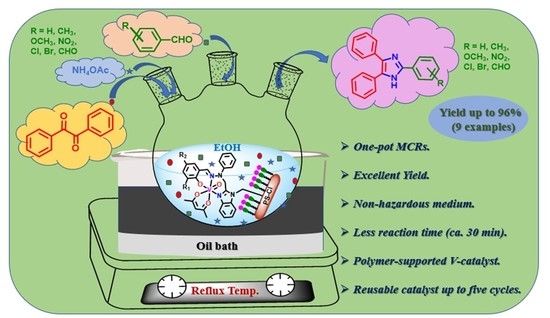
Polymer-Supported Oxidovanadium(IV) Complexes and Their Catalytic Applications in One-Pot Multicomponent Reactions Producing Biologically Active 2,4,5-Trisubstituted-1H-imidazoles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterization of Ligands
2.2. Synthesis and Solid-State Characterization of Complexes
2.3. Thermal Study
2.4. Structure Description of HL1 (I) and [VIVO(acac)L1] (1)
2.5. IR Spectral Study
2.6. UV-Visible Spectral Study
2.7. EPR Spectral Study
2.8. Powder-X-ray Diffraction Analysis
2.9. Field Emission-Scanning Electron Microscopy (FE-SEM) and Energy Dispersive X-ray Analysis (EDS)
2.10. Atomic Force Microscopic Study
2.11. Catalytic Activity Study: Synthesis of 2,4,5-Triphenyl-1H-Imidazole and Its Derivatives
2.12. Scope of the MCR to Other Lophine Derivatives
2.13. Regeneration of the Supported Catalyst and Study of Recyclability and Stability
2.14. Comparison of the Catalytic Efficiency of Catalyst 3 with the Literature Data
2.15. Reactivity of Complex 1 with Multicomponent Reagents and a Possible Reaction Mechanism
2.16. Mechanistic Study of the Polymer-Supported Heterogeneous Oxidovanadium(IV) Catalyst in the Synthesis of Lophine Derivatives
3. Experimental Section
3.1. Materials, Instrumentation and Characterization Procedures
3.2. Synthesis of Ligand HL1 (I) and HL2 (II)
3.3. Synthesis of [VIVO(acac)L1] (1)
3.4. Synthesis of [VIVO(acac)L2] (2)
3.5. Synthesis of [VIVO(acac)L1]@PS (3)
3.6. Synthesis of [VIVO(acac)L2]@PS (4)
3.7. Catalytic Activity—Synthesis of Lophine ((2,4,5-Triphenyl-1H-imidazole)) Derivatives Using the One-Pot Multicomponent Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellina, F.; Cauteruccio, S.; Rossi, R. Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles. Tetrahedron 2007, 63, 4624–4751. [Google Scholar] [CrossRef]
- Nagarapu, L.; Aneesa Satyender, A.; Chandana, G.; Bantu, R. Synthesis and antimicrobial activity of novel analogs of trifenagrel. J. Heterocycl. Chem. 2009, 46, 195–200. [Google Scholar] [CrossRef]
- Fallah, N.S.; Mokhtary, F.M. Tin oxide nanoparticles (SnO2-NPs): An efficient catalyst for the one-pot synthesis of highly substituted imidazole derivatives. J. Taibah Univ. Sci. 2015, 9, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Choghamarani, A.G.; Hajjami, M.; Gholamian, F.; Azadi, G. Aspartic acid as a highly efficient and nontoxic organocatalyst for the one-pot synthesis of tri- and tetrasubstituted imidazoles under solvent-free conditions. Russ. J. Org. Chem. 2015, 51, 352–356. [Google Scholar] [CrossRef]
- Kamijo, S.; Yamamoto, Y. Recent progress in the catalytic synthesis of imidazoles. Chem. Asian J. 2007, 2, 568–578. [Google Scholar] [CrossRef]
- Das, U.K.; Shimonb, L.J.W.; Milstein, D. Imidazole synthesis by transition metal free, base-mediated deaminative coupling of benzylamines and nitriles. Chem. Commun. 2017, 53, 13133–13136. [Google Scholar] [CrossRef]
- Jin, Z. Imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2006, 23, 464–496. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Camp, J.E. Recent advances in the synthesis of imidazoles. Org. Biomol. Chem. 2020, 18, 3950–3964. [Google Scholar] [CrossRef]
- Koga, H.; Nanjoh, Y.; Makimura, K.; Tsuboi, R. In vitro antifungal activities of luliconazole, a new topical imidazole. Med. Mycol. J. 2009, 47, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Sennequier, N.; Wolman, D.; Stueh, D.J. Antifungal imidazoles block assembly of inducible NO synthase into an active dimer. J. Biol. Chem. 1999, 274, 930–938. [Google Scholar] [CrossRef] [Green Version]
- Aswathy, V.V.; Hayta, S.A.; Yalcin, G.; Marya, Y.S.; Panicker, C.Y.; Jojo, P.J.; Onurdag, F.K.; Armakovic, S.; Armakovic, A.J.; Yildiz, I.; et al. Modification of benzoxazole derivative by bromine-spectroscopic, antibacterial and reactivity study using experimental and theoretical procedures. J. Mol. Struct. 2017, 1141, 495–511. [Google Scholar] [CrossRef]
- Khan, M.S.; Siddiqui, S.A.; Siddiqui, M.S.; Goswami, U.; Srinivasan, K.V.; Khan, M.I. Antibacterial activity of synthesized 2,4,5-trisubstituted imidazole derivatives. Chem. Biol. Drug Des. 2008, 72, 197–204. [Google Scholar] [CrossRef]
- Kumar, N.; Bhatnagar, A.; Dudhe, R. Synthesis of 3-(4, 5-dihydro-1-phenyl-5-substituted phenyl-1H-pyrazol-3-yl)-2H-chromen-2-one derivatives and evaluation of their anticancer activity. Arab. J. Chem. 2017, 10, 2443–2452. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; LaRosa, C.; Antwi, J.; Govindarajan, R.; Werbovetz, K.A. Imidazoles as potential anticancer agents: An update on recent studies. Molecules 2021, 26, 4213. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, M.; Zhang, L.; Lei, Z.; Chen, C.; Zhang, T.; Chen, L.; Sun, J. Discovery of novel celastrol−imidazole derivatives with anticancer activity in vitro and in vivo. J. Med. Chem. 2022, 65, 4578–4589. [Google Scholar] [CrossRef]
- Lee, J.C.; Laydon, J.T.; McDonnell, P.C.; Gallagher, T.F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M.J.; Keys, J.R.; Land, S.W.; et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994, 372, 739–746. [Google Scholar] [CrossRef]
- Hossain, M.; Nanda, A.K. A review on heterocyclic: Synthesis and their application in medicinal chemistry of imidazole moiety. Chem. Sci. J. 2018, 6, 83–94. [Google Scholar] [CrossRef]
- Stella, P.C.R.; Rajam, S.; Venkatraman, B.R. Synthesis, characterization and biological evaluation of benzoxazole derivatives. J. Chem. Pharm. Res. 2012, 4, 2988–2993. [Google Scholar]
- De, D.; Halder, D.; Shin, I.; Kim, K.K. Small molecule-induced cellular conversion. Chem. Soc. Rev. 2017, 46, 6241–6254. [Google Scholar] [CrossRef]
- Asressu, K.H.; Chan, C.K.; Wang, C.C. TMSOTf-catalyzed synthesis of trisubstituted imida-zoles using hexamethyl disilazane as a nitrogen source under neat and microwave irradiation conditions. RSC Adv. 2021, 11, 28061–28071. [Google Scholar] [CrossRef]
- Serrao, E.; Xu, Z.L.; Debnath, B.; Christ, F.; Debyser, Z.; Long, Y.Q.; Neamati, N. Discovery of a novel 5-carbonyl-1H-imidazole-4-carboxamide class of inhibitors of the HIV-1 integrase-LEDGF/p75 interaction. Bioorg. Med. Chem. 2013, 21, 5963–5972. [Google Scholar] [CrossRef] [Green Version]
- Kamel, M.M.; Ali, H.I.; Anwar, M.M.; Mohamed, N.A.; Soliman, A.M.M. Synthesis, antitumor activity and molecular docking study of novel sulfonamide-schiff’s bases, thiazolidinones, benzothiazinones and their C-nucleoside derivatives. Eur. J. Med. Chem. 2010, 45, 572–580. [Google Scholar] [CrossRef]
- Wierzbicki, A.S. Imidazole derivatives as cholesterol-lowering agents. Int. J. Cardiol. 2003, 90, 145–146. [Google Scholar] [CrossRef]
- Jasim, S.H.; Sheikha, G.M.A.; Abuzaid, H.M.; Al-Qirim, T.M.; Shattat, G.F.; Sabbah, D.A.; Ala, S.A.; Aboumair, M.S.; Sweidan, K.A.; Bkhaitan, M.M. Synthesis and in vivo lipid-lowering activity of novel imidazoles-5-carboxamide derivatives in triton-WR-1339-induced hyperlipidemic wistar rats. Chem. Pharm. Bull. 2018, 66, 953–958. [Google Scholar] [CrossRef] [Green Version]
- Rad, M.S.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Rad, J.S. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar]
- Katsura, Y.; Nishino, S.; Takasugi, H. Studies on antiulcer drugs. II. Synthesis and antiuler activities of imidazo[1,2-a]pyridinyl-2-oxobenzoxazolidines-3-oxo-2H-1, 4-benzoxazines and related compounds. Chem. Pharm. Bull. 1991, 39, 2937–2943. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, A.; Lassen, P.B.; Henricsson, M.; Wu, H.; Adriouch, S.; Belda, E.; Chakaroun, R.; Nielsen, T.; Bergh, P.O.; Rouault, C.; et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020, 11, 5881. [Google Scholar] [CrossRef]
- Asgari, M.S.; Khanaposhtani, M.M.; Sharaf, Z.; Faramarzi, M.A.; Rastegar, H.; Esfahani, E.N.; Bandarian, F.; Rashidi, P.R.; Rahimi, R.; Biglar, M.; et al. Design and synthesis of 4,5-diphenyl-imidazol-1,2,3-triazole hybrids as new anti-diabetic agents: In vitro α-glucosidase inhibition, kinetic and docking studies. Mol. Divers. 2021, 25, 877–888. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Synthesis and biological activities of local anesthetics. RSC Adv. 2019, 9, 41173–41191. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.A.; Fathi, Z.; Achebe, F.; Akuche, C.; Brown, S.E.; Choi, S.; Fan, J.; Jenkins, S.; Kluender, H.C.E.; Konkar, A.; et al. Optimization of imidazole amide derivatives as cannabinoid-1 receptor antagonists for the treatment of obesity. Bioorg. Med. Chem. Lett. 2007, 7, 2706–2711. [Google Scholar] [CrossRef]
- Almeer, R.S.; Hammad, S.F.; Leheta, O.F.; Moneim, A.E.A.; Amin, H.K. Anti-inflammatory and anti-hyperuricemic functions of two synthetic hybrid drugs with dual biological active sites. Int. J. Mol. Sci. 2019, 20, 5635. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, N.; Palne, S.; Gupta, S.; Bhandari, K. Aryloxy cyclohexyl imidazoles: A novel class of antileishmanial agents. Bioorg. Med. Chem. Lett. 2009, 19, 324–327. [Google Scholar] [CrossRef]
- El-Shahat, M.; El-Sofany, W.; Soliman, A.G.A.; Hasanin, M. Newly synthesized imidazolo- triazole, imidazolotriazine, and imidazole-pyrazole hybrid derivatives as promising antimicrobial agents. J. Mol. Struct. 2022, 1250, 131727. [Google Scholar] [CrossRef]
- Gupta, G.K.; Kumar, V.; Kaur, K. Imidazole containing natural products as antimicrobial agents: A review. Nat. Prod. J. 2014, 4, 73–81. [Google Scholar] [CrossRef]
- Perchellet, E.M.; Perchellet, J.P.; Baures, P.W. Imidazole-4,5-dicarboxamide derivatives with antiproliferative activity against HL-60 cells. J. Med. Chem. 2005, 48, 5955–5965. [Google Scholar] [CrossRef]
- Chen, J.; Ahn, S.; Wang, J.; Lu, Y.; Dalton, J.T.; Miller, D.D.; Li, W. Discovery of novel 2-aryl-4-benzoyl-imidazole (ABI-III) analogues targeting tubulin polymerization as antiproli-ferative agents. J. Med. Chem. 2012, 55, 7285–7289. [Google Scholar] [CrossRef] [Green Version]
- Sarkarzadeh, H.; Miri, R.; Firuzi, O.; Amini, M.; Razzaghi-Asl, N.; Edraki, N.; Shafiee, A. Synthesis and antiproliferative activity evaluation of imidazole-based indeno[1,2-b]quinoline-9,11-dione derivatives. Arch. Pharm. Res. 2013, 36, 436–447. [Google Scholar] [CrossRef]
- Magnus, N.A.; Diseroad, W.D.; Nevill, C.R.; Wepsiec, J.P. Synthesis of imidazole based p38 MAP (mitogen-activated protein) kinase inhibitors under buffered conditions. Org. Process Res. Dev. 2006, 10, 556–560. [Google Scholar] [CrossRef]
- Szabo, B. Imidazoline antihypertensive drugs: A critical review on their mechanism of action. Pharmacol. Ther. 2002, 93, 1–35. [Google Scholar] [CrossRef]
- Siwach, A.; Verma, P.K. Synthesis and therapeutic potential of imidazole containing compounds. BMC Chem. 2021, 15, 12. [Google Scholar] [CrossRef]
- Chopra, P.N.; Sahu, J.K. Biological significance of imidazole-based analogues in new drug development. Curr. Drug Discov. Technol. 2020, 17, 574–584. [Google Scholar] [CrossRef]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem. Biol. Drug Des. 2015, 86, 19–65. [Google Scholar] [CrossRef]
- Burungale, S.D.; Bhitre, M.J. Synthesis of 2,4,5-triphenyl imidazole derivatives and biological evaluation for their antibacterial and antiinflammatory activity. Int. J. Pharm. Sci. Res. 2013, 4, 4051–4057. [Google Scholar]
- Bourissou, D.; Guerret, O.; Gabbai, F.P.; Bertrand, G. Stable carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Liddle, S.T. F-block N-heterocyclic carbene complexes. Chem. Commun. 2006, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- De-souza, R.F.; Suarez, P.A.Z. Ionic liquid (molten salt) phase organometallic catalysis jairton dupont. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar]
- Nara, S.J.; Naik, P.U.; Harjani, J.R.; Salunkhe, M.M. Potential of ionic liquids in greener methodologies involving biocatalysis and other synthetically important transformations. Indian J. Chem. 2006, 45, 2257–2269. [Google Scholar] [CrossRef]
- Yao, W.; Wang, J.; Zhong, A.; Wang, S.; Shao, Y. Transition-metal-free catalytic hydro boration reduction of amides to amines. Org.Chem. Front. 2020, 7, 3515–3520. [Google Scholar] [CrossRef]
- Yang, Q.; Wen, Y.; Zhong, A.; Xu, J.; Shao, S. An HBT-based fluorescent probe for nitro reductase determination and its application in Escherichia coli cell imaging. New J. Chem. 2020, 44, 16265–16268. [Google Scholar] [CrossRef]
- Yao, W.; Fang, H.; He, Q.; Peng, D.; Liu, G.; Huang, Z. A BEt3-base catalyst for amide reduction with silane. J. Org. Chem. 2019, 84, 6084−6093. [Google Scholar] [CrossRef]
- Yao, W.; Wang, J.; Lou, Y.; Wu, H.; Qi, X.; Yanga, J.; Zhong, A. Chemoselective hydro borative reduction of nitro motifs using a transition-metal-free catalyst. Org. Chem. Front. 2021, 8, 4554–4559. [Google Scholar] [CrossRef]
- Munnik, P.; Jongh, P.E.d.; Jong, K.P.d. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, G. Conducting polymer-based catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keefee, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Meeuwissen, J.; Reek, J.N. Supramolecular catalysis beyond enzyme mimics. Nat. Chem. 2010, 2, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Narkhede, U.C.; Palimkar, S.S.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Room temperature ionic liquid promoted improved and rapid synthesis of 2,4,5-triaryl imidazoles from aryl aldehydes and 1,2-diketones or -hydroxyketone. Tetrahedron 2005, 61, 3569. [Google Scholar] [CrossRef]
- Shaabani, A.; Rahmati, A. Silica sulfuric acid as an efficient and recoverable catalyst for the synthesis of trisubstituted imidazoles. J. Mol. Catal. A Chem. 2006, 249, 246. [Google Scholar] [CrossRef]
- Das, S.S.; Hazarika, P.; Konwar, D. An efficient and one pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by InCl3·3H2O. Tetrahedron Lett. 2008, 49, 2216–2220. [Google Scholar]
- Samai, S.; Nandi, G.C.; Singh, P.; Singh, M. L-Proline: An efficient catalyst for the one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron 2009, 65, 10155–10161. [Google Scholar] [CrossRef]
- Murthy, S.N.; Madhav, B.; Nageswar, Y.V.D. DABCO as a mild and efficient catalytic system for the synthesis of highly substituted imidazoles via multi-component condensation strategy. Tetrahedron Lett. 2010, 51, 5252–5257. [Google Scholar] [CrossRef]
- Teimouri, A.; Chermahini, A.N. An efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed via solid acid nano-catalyst. J. Mol. Catal. A Chem. 2011, 346, 39–45. [Google Scholar] [CrossRef]
- Zarnegar, Z.; Safari, J. Catalytic activity of Cu nanoparticles supported on Fe3O4-polyethylene glycol nanocomposites for the synthesis of substituted imidazoles. New J. Chem. 2014, 38, 4555–4565. [Google Scholar] [CrossRef]
- Maleki, B.; Eshghi, H.; Khojastehnezhad, A.; Tayebee, R.; Ashrafi, S.S.; Kahooa, G.E.; Moeinpourc, F. Silica coated magnetic NiFe2O4 nanoparticle supported phosphomolybdic acid; synthesis, preparation and its application as a heterogeneous and recyclable catalyst for the one-pot synthesis of tri- and tetra-substituted imidazoles under solvent free conditions. RSC Adv. 2015, 5, 64850–64857. [Google Scholar]
- Ran, Y.; Li, M.; Zhang, Z.Z. β–cyclodextrin–propyl sulfonic acid catalysed one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles as local anesthetic agents. Molecules 2015, 20, 20286–20296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaabani, A.; Afshari, R.; Hooshmand, S.E. Crosslinked chitosan nanoparticle-anchored magnetic multi-wall carbon nanotubes: A bio-nanoreactor with extremely high activity toward click-multi-component reactions. New J. Chem. 2017, 41, 8469–8481. [Google Scholar] [CrossRef]
- Shaabani, A.; Afshari, R.; Hooshmand, S.E.; Nejad, M.K. Molecularly imprinted polymer as an eco-compatible nanoreactor in multicomponent reactions: A remarkable synergy for expedient access to highly substituted imidazoles. ACS Sustain. Chem. Eng. 2017, 5, 9506–9516. [Google Scholar] [CrossRef]
- Sonar, J.; Pardeshi, S.; Dokhe, S.; Pawar, R.; Kharat, K.; Zine, A.; Matsagar, B.; Wu, K.; Thore, S. An efcient method for the synthesis of 2,4,5-trisubstituted imidazoles using lactic acid as promoter. SN Appl. Sci. 2019, 1, 1045. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Le, N.P.T.; Nguyen, T.T.; Tran, P.H. An efficient multicomponent synthesis of 2,4,5- trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by a magnetic nanoparticle supported Lewis acidic deep eutectic solvent. RSC Adv. 2019, 9, 38148–38153. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh, Z.; Radinekiyan, F.; Eivazzadeh-keihan, R.; Maleki, A. Development of novel and green NiFe2O4/geopolymer nanocatalyst based on bentonite for synthesis of imidazole heterocycles by ultrasonic irradiations. Sci. Rep. 2020, 10, 11671. [Google Scholar] [CrossRef]
- Heidari, M.; Rezaei, M.; Badri, R. Tributylhexadecylphosphonium bromide: An efficient reagent system for the one-pot synthesis of 2,4,5-trisubstituted imidazoles. Heterocycl. Commun. 2015, 21, 73–76. [Google Scholar] [CrossRef]
- Kermanizadeh, S.; Naeimi, H.; Mousavi, S. An efficient and eco-compatible multicomponent synthesis of 2,4,5-trisubstituted imidazole derivatives using modified-silica-coated cobalt ferrite nanoparticles with tungstic acid. Dalton Trans. 2023, 52, 1257–1267. [Google Scholar] [CrossRef]
- Ahmadi, L.K.; Khademini, S.; Marjani, A.P.; Nozad, E. Microwave-assisted preparation of polysubstituted imidazoles using Zingiber extract synthesized green Cr2O3 nanoparticles. Sci. Rep. 2022, 12, 19942. [Google Scholar] [CrossRef] [PubMed]
- Mato-López, A.; Sar-Rañó, M.; Riopedre-Fernández, M.L.; Díaz-Prado, A.; Gil, Á.; Sánchez-González, N.; Fernández-Bertólez, J.; Méndez, V.; Valdiglesias, F.A. Relationship between structure and cytotoxicity of vanadium and molybdenum complexes with pyridoxal derived ligands. J. Inorg. Biochem. 2022, 235, 11937. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Maji, A.; Mohanty, A.; Ghosh, K. Design and synthesis of base-metal nickel(II) based catalysts: Studies on nearly selective formation of 1-butene from ethylene. J. Organomet. Chem. 2021, 934, 121631. [Google Scholar] [CrossRef]
- Maurya, M.R.; Kumar, A.; Ebel, M.; Rehder, D. Synthesis, characterization, reactivity, and catalytic potential of model vanadium (IV, V) complexes with benzimidazole-derived ONN donor ligands. Inorg. Chem. 2006, 45, 5924–5937. [Google Scholar] [CrossRef] [PubMed]
- Adao, P.; Maurya, M.R.; Kumar, U.; Avecilla, F.; Henriques, R.T.; Kusnetsov, M.L.; Pessoa, J.C.; Correia, I. Vanadium-salen and -salan complexes: Characterization and application in oxygen transfer reaction. Pure Appl. Chem. 2009, 81, 1279–1296. [Google Scholar] [CrossRef]
- Maurya, M.R.; Chauhan, A.; Arora, S.; Gupta, P. Triazole based oxidovanadium(V) complex supported on chloromethylated polymer and its catalytic activity for the synthesis of dihydropyrimidinones (DHPMs). Catal. Today 2022, 3, 397–399. [Google Scholar] [CrossRef]
- Maurya, M.R.; Chaudhary, N.; Avecilla, F.; Adão, P.; Pessoa, J.C. Oxidovanadium(IV) and dioxidovanadium(V) complexes of hydrazones of 2-benzoylpyridine and their catalytic applications. Dalton Tans. 2015, 44, 1211–1232. [Google Scholar] [CrossRef]
- Tshentu, Z.R.; Togo, C.; Walmsley, R.S. Polymer-anchored oxovanadium(IV) complex for the oxidation of thioanisole, styrene and ethylbenzene. J. Mol. Catal. A 2010, 318, 30–35. [Google Scholar] [CrossRef]
- Ogunlaja, A.S.; Chidawanyika, W.; Antunes, E.; Fernandes, M.A.; Nyokong, T.; Torto, N.; Tshentu, Z.R. Oxovanadium(IV)-catalysed oxidation of dibenzothiophene and 4,6-dimethyl dibenzothiophene. Dalton Trans. 2012, 41, 13908–13918. [Google Scholar] [CrossRef]
- Bai, Y.B.; Zhang, A.L.; Tang, J.J.; Gao, J.M. Synthesis and antifungal activity of 2-chloro methyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro. J. Agric. Food Chem. 2013, 61, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Jiao, J.; Wang, X.B.; Chen, M.; Fu, X.C.; Si, W.J.; Yang, C.L. Synthesis, Characterization, and antifungal activity of novel benzo[4,5]imidazo[1,2-d][1,2,4]triazine derivatives. Molecules 2018, 23, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, R.A.; Jones, M.M. Vanadium(IV)oxy(acetylacetonate). Inorg. Synth. 1957, 5, 113–116. [Google Scholar]
- Maurya, M.R.; Patter, A.; Singh, D.; Ghosh, K. Polymer-supported dioxidovanadium(V) complex-based heterogeneous catalyst for multicomponent Biginelli reaction producing biologically active 3,4-dihydropyrimidin-2-(1H)-ones. Catalysts 2023, 13, 234. [Google Scholar] [CrossRef]



























| Entry | Compound | Solvent | λmax/nm (ε/M−1 cm−1) |
|---|---|---|---|
| 1 | HL1 (I) | DMSO | 258 (1.80 × 103), 277 (1.35 × 103), 283 (1.52 × 103), 299 (1.10 × 103), 346 (2.65 × 103) |
| 2 | HL2 (II) | DMSO | 256 (1.96 × 103), 277 (1.31 × 103), 283 (1.54 × 103), 344 (2.53 × 103) |
| 3 | [VIVO(acac)L1] (1) | DMSO | 258 (3.06 × 103), 277 (2.79 × 103), 283 (2.71 × 103), 306 (1.81× 103), 346 (1.91 × 103), 403 (0.38 × 103) |
| 4 | [VIVO(acac)L2] (2) | DMSO | 258 (2.96 × 103), 275 (2.54 × 103), 282 (2.58 × 103), 342 (0.61 × 103), 414 (0.47 × 103) |
| 5 | [VIV(acac)L1]@PS (3) | Nujol | 230, 272, 353, 409 |
| 6 | [VIV(acac)L2]@PS (4) | Nujol | 232, 289, 356, 409 |
| Compound | gx, gy | |Ax|, |Ay| (×10−4 cm−1) | gz | |Az| (×10−4 cm−1) |
|---|---|---|---|---|
| [VIVO(acac)(L1)] (1) | 1.970 | 89.9 | 1.945 | 184.4 |
| [VIVO(acac)(L2)] (2) | 1.968 | 86.6 | 1.951 | 181.2 |
| [VIVO(acac)L1]@PS (3) | 1.971 | 72.71 | 1.945 | 170.4 |
| [VIVO(acac)L2]@PS (4) | 1.969 | 70.74 | 1.952 | 168.38 |
| Sl. No. | Compound | Size (μm2) | Average Surface Roughness (nm) a |
|---|---|---|---|
| 1 | PS-Cl | 10 × 10 | 45.1 |
| 2 | [VIVO(acac)L1]@PS (3) | 10 × 10 | 16.1 |
| 3 | [VIVO(acac)L2]@PS (4) | 10 × 10 | 13.1 |
| 4 | [VIVO(acac)L1]@PS (3) (recycled) | 10 × 10 | 16.2 |
| Entry | Catalyst (g) | Solvent (10 mL) | Time (min) | Temp. (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.015 | MeCN | 15 | 80 | 69 |
| 2 | 0.015 | THF | 15 | Reflux | 63 |
| 3 | 0.015 | CHCl3 | 15 | Reflux | 17 |
| 4 | 0.015 | EtOAc | 15 | Reflux | 64 |
| 5 | 0.015 | H2O | 15 | 80 | 58 |
| 6 | 0.015 | EtOH:H2O (V/V) | 15 | 80 | 81 |
| 7 | 0.015 | EtOH | 15 | Reflux | 89 |
| 8 | 0.015 | EtOH | 15 | 70 | 79 |
| 9 | 0.015 | EtOH | 15 | 60 | 65 |
| 10 | 0.015 | EtOH | 15 | 50 | 51 |
| 11 | 0.015 | EtOH | 15 | 40 | 37 |
| 12 a | 0.015 | EtOH | 30 | Reflux | 91 |
| 13 | 0.015 | EtOH | 45 | Reflux | 92 |
| 14 | 0.05 | EtOH | 60 | Reflux | 71 |
| 15 | 0.010 | EtOH | 60 | Reflux | 83 |
| 16 | 0.015 | EtOH | 60 | Reflux | 91 |
| 17 | 0.020 | EtOH | 60 | Reflux | 92 |
| Entry No. | Catalyst and Conditions | Reaction Time (Minutes) | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | [Hbim] BF4 (4 mmol)/solvent free/100 °C | 60 | 95 | [56] |
| 2 | Silica Sulfuric acid (500 mg)/water/reflux | 240 | 73 | [57] |
| 3 | InCl3·3H2O/MeOH/rt | 500 | 82 | [58] |
| 4 | L-proline/MeOH/rt | 540 | 90 | [59] |
| 5 | DABCO (0.7 mol%)/t-BuOH/65 °C | 45 | 97 | [60] |
| 6 | Nontmorilonite/EtOH/reflux | 90 | 70 | [61] |
| 7 | Fe3O4-PEG-Cu/solvent free/110 °C | 30 | 98 | [62] |
| 8 | Scolecite (2 wt%)/lactic acid/160 °C | 180 | 90 | [67] |
| 9 | NFS-PMA (20 mg)/solvent free/120 °C | 20 | 94 | [63] |
| 10 | -CD-PSA (2 mol%)/solvent free/100 °C | 20 | 96 | [64] |
| 11 | CSNP/MWCNT@Fe3O4/EtOH/reflux | 60 | 86 | [65] |
| 12 | MIP Nanoreactors/solvent free/120 °C | 20 | 97 | [66] |
| 13 | LADES@MNP/solvent free/sonication | 120 | 83 | [68] |
| 14 | TBHDPB (5 mol%)/EtOH/reflux | 60 | 85 | [70] |
| 15 | CoFe2O4@SiO2@(CH2)3OWO3H NPs(10 mg)/solvent free/110 °C | 20 | 87 | [71] |
| 16 | V catalyst 1 (0.62 mg)/EtOH/reflux | 30 | 93 | This work |
| 17 | V catalyst 3 (15 mg)/EtOH/reflux | 30 | 91 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurya, M.R.; Nandi, M.; Patter, A.; Avecilla, F.; Ghosh, K. Polymer-Supported Oxidovanadium(IV) Complexes and Their Catalytic Applications in One-Pot Multicomponent Reactions Producing Biologically Active 2,4,5-Trisubstituted-1H-imidazoles. Catalysts 2023, 13, 615. https://doi.org/10.3390/catal13030615
Maurya MR, Nandi M, Patter A, Avecilla F, Ghosh K. Polymer-Supported Oxidovanadium(IV) Complexes and Their Catalytic Applications in One-Pot Multicomponent Reactions Producing Biologically Active 2,4,5-Trisubstituted-1H-imidazoles. Catalysts. 2023; 13(3):615. https://doi.org/10.3390/catal13030615
Chicago/Turabian StyleMaurya, Mannar R., Monojit Nandi, Akhil Patter, Fernando Avecilla, and Kaushik Ghosh. 2023. "Polymer-Supported Oxidovanadium(IV) Complexes and Their Catalytic Applications in One-Pot Multicomponent Reactions Producing Biologically Active 2,4,5-Trisubstituted-1H-imidazoles" Catalysts 13, no. 3: 615. https://doi.org/10.3390/catal13030615
APA StyleMaurya, M. R., Nandi, M., Patter, A., Avecilla, F., & Ghosh, K. (2023). Polymer-Supported Oxidovanadium(IV) Complexes and Their Catalytic Applications in One-Pot Multicomponent Reactions Producing Biologically Active 2,4,5-Trisubstituted-1H-imidazoles. Catalysts, 13(3), 615. https://doi.org/10.3390/catal13030615