Preparation of Co-CNK-OH and Its Performance in Fenton-like Photocatalytic Degradation of Tetracycline
Abstract
:1. Introduction
2. Results
2.1. Characterization of As-Prepared Samples
2.2. Morphology and Structural Properties
2.3. Optical and Photoelectric Properties
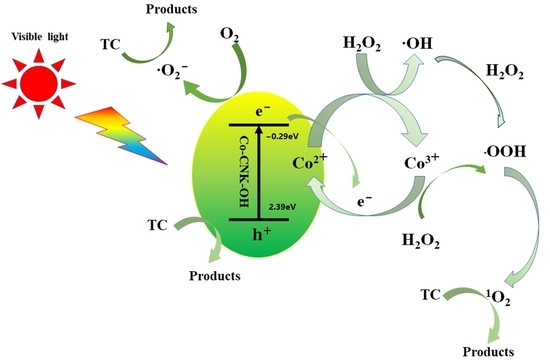
2.4. Catalytic Performance and Mechanism of As-Prepared Samples
3. Materials and Methods
3.1. Chemicals
3.2. Materials Synthesis
3.2.1. Preparation of CNK-OH
3.2.2. Fabrication of Co-CNK-OH Composites
3.3. Degradation Experiments
3.4. TC Mineralization Experiment
3.5. Trapping Test of Active Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Li, W.B.; Kumar, V.; Necibi, M.C.; Mu, Y.J.; Shi, C.Z.; Chaurasia, D.; Chauhan, S.; Chaturvedi, P.; Sillanpaa, M.; et al. Synthetic organic antibiotics residues as emerging contaminants waste-to-resources processing for a circular economy in China: Challenges and perspective. Environ. Res. 2022, 211, 113075. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Q.; Zhang, Y.; Li, Y.; Yang, X.; Zheng, H.; Chen, L.; Li, F. Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water Res. 2022, 210, 117985. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Huo, P.W.; Luo, Y.Y.; Liu, X.L.; Wu, D.; Gao, X.; Li, C.X.; Yan, Y.S. Performance of molecularly imprinted photocatalysts based on fly-ash cenospheres for selective photodegradation of single and ternary antibiotics solution. J. Mol. Catal. A-Chem. 2013, 378, 91–98. [Google Scholar] [CrossRef]
- Sun, S.; Geng, J.; Ma, L.; Sun, X.; Qi, H.; Wu, Y.; Zhang, R. Changes in antibiotic resistance genotypes and phenotypes after two typical sewage disposal processes. Chemosphere 2022, 291 Pt 2, 132833. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernandez-Calvino, D.; Nunez-Delgado, A.; Fernandez-Sanjurjo, M.J.; Arias-Estevez, M.; Alvarez-Rodriguez, E. Estimation of adsorption/desorption Freundlich's affinity coefficients for oxytetracycline and chlortetracycline from soil properties: Experimental data and pedotransfer functions. Ecotoxicol. Environ. Saf. 2020, 196, 110584. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.X.; Zhang, H.M.; Feng, Y.J.; Yang, F.L.; Zhang, J.P. Removal of trace antibiotics from wastewater: A systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Liu, M.; Xia, H.; Yang, W.; Liu, X.; Xiang, J.; Wang, X.; Hu, L.; Lu, F. Novel Cu-Fe bi-metal oxide quantum dots coupled g-C3N4 nanosheets with H2O2 adsorption-activation trade-off for efficient photo-Fenton catalysis. Appl. Catal. B Environ. 2022, 301, 120765. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Qian, X.; Wu, Y.; Kan, M.; Fang, M.; Yue, D.; Zeng, J.; Zhao, Y. FeOOH quantum dots coupled g-C3N4 for visible light driving photo- Fenton degradation of organic pollutants. Appl. Catal. B Environ. 2018, 237, 513–520. [Google Scholar] [CrossRef]
- Li, Y.; Luo, N.; Tian, Z.; Li, H.; Yang, M.; Shang, W.; Shen, Y.; Qu, M.; Zhou, A. H2O2-free photo-Fenton degradation of organic pollutants on thermally exfoliated g-C3N4. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 586, 124190. [Google Scholar] [CrossRef]
- Cui, K.P.; Yang, T.T.; Chen, Y.H.; Weerasooriya, R.; Li, G.H.; Zhou, K.; Chen, X. Magnetic recyclable heterogeneous catalyst Fe3O4/g-C3N4 for tetracycline hydrochloride degradation via photo-Fenton process under visible light. Environ. Technol 2022, 43, 3341–3354. [Google Scholar] [CrossRef]
- Lu, N.; Liu, N.; Zhang, C.; Su, Y.; Shang, K.; Jiang, N.; Li, J.; Wu, Y. CO2 conversion promoted by potassium intercalated g-C3N4 catalyst in DBD plasma system. Chem. Eng. J. 2021, 417, 129283. [Google Scholar] [CrossRef]
- Lu, Z.-Z.; Li, S.-Q.; Xiao, J.-Y. Synergetic effect of Na-Ca for enhanced photocatalytic performance in NOx degradation by g-C3N4. Catal. Lett. 2021, 151, 370–381. [Google Scholar] [CrossRef]
- Zhou, M.; Jing, L.; Dong, M.; Lan, Y.; Xu, Y.; Wei, W.; Wang, D.; Xue, Z.; Jiang, D.; Xie, J. Novel broad-spectrum-driven g-C3N4 with oxygen-linked band and porous defect for photodegradation of bisphenol A, 2-mercaptophenthiazole and ciprofloxacin. Chemosphere 2021, 268, 128839. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, Y.; Deng, F.; Wang, M.; Chen, D. Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction with excellent Fenton-like photocatalytic activity for efficient degradation of pharmaceutical pollutants. Sep. Purif. Technol. 2020, 246, 116890. [Google Scholar] [CrossRef]
- Lu, X.; Jin, Y.; Zhang, X.; Xu, G.; Wang, D.; Lv, J.; Zheng, Z.; Wu, Y. Controllable synthesis of graphitic C3N4/ultrathin MoS2 nanosheet hybrid nanostructures with enhanced photocatalytic performance. Dalton. Trans. 2016, 45, 15406–15414. [Google Scholar] [CrossRef]
- Thi Quyen, V.; Jae Kim, H.; Kim, J.; Thi Thu Ha, L.; Thi Huong, P.; My Thanh, D.; Minh Viet, N.; Quang Thang, P. Synthesizing S-doped graphitic carbon nitride for improvement photodegradation of tetracycline under solar light. Sol. Energy 2021, 214, 288–293. [Google Scholar] [CrossRef]
- Bao, J.; Bai, W.; Wu, M.; Gong, W.; Yu, Y.; Zheng, K.; Liu, L. Template-mediated copper doped porous g-C3N4 for efficient photodegradation of antibiotic contaminants. Chemosphere 2022, 293, 133607. [Google Scholar] [CrossRef]
- Zhou, M.; Dong, G.; Yu, F.; Huang, Y. The deep oxidation of NO was realized by Sr multi-site doped g-C3N4 via photocatalytic method. Appl. Catal. B Environ. 2019, 256, 117825. [Google Scholar] [CrossRef]
- Wu, X.; Chen, F.; Wang, X.; Yu, H. In situ one-step hydrothermal synthesis of oxygen-containing groups-modified g-C3N4 for the improved photocatalytic H2-evolution performance. Appl. Surf. Sci. 2018, 427, 645–653. [Google Scholar] [CrossRef]
- Shi, Y.X.; Li, L.L.; Xu, Z.; Sun, H.R.; Guo, F.; Shi, W.L. One-step simple green method to prepare carbon-doped graphitic carbon nitride nanosheets for boosting visible-light photocatalytic degradation of tetracycline. J. Chem. Technol. Biotechnol. 2021, 96, 3122–3133. [Google Scholar] [CrossRef]
- Wu, X.; Gao, D.; Wang, P.; Yu, H.; Yu, J. NH4Cl-induced low-temperature formation of nitrogen-rich g-C3N4 nanosheets with improved photocatalytic hydrogen evolution. Carbon 2019, 153, 757–766. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Z.-T.; Andrew Lin, K.-Y.; Wei, W.-H.; Wang, K.-H. Synergistic effect of KCl mixing and melamine/urea mixture in the synthesis of g-C3N4 for photocatalytic removal of tetracycline. J. Ind. Eng. Chem. 2022, 107, 118–125. [Google Scholar] [CrossRef]
- Song, H.; Liu, L.; Wang, H.; Feng, B.; Xiao, M.; Tang, Y.; Qu, X.; Gai, H.; Huang, T. Adjustment of the band gap of Co-doped KCl/NH4Cl/g-C3N4 for enhanced photocatalytic performance under visible light. Mater. Sci. Semicond. Process. 2021, 128, 105757. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, X.; Zheng, Q.; Zhang, Y.; Lv, S.W. The calcium alginate-immobilized Co-g-C3N4 composite microspheres as an efficient mediator to activate peroxymonosulfate for degrading organic pollutants. Environ. Res 2022, 215 Pt 2, 114414. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Fang, W.Q.; Wang, H.F.; Zhang, H.; Zhao, H.; Yao, Y.; Yang, H.G. Surface hydrogen bonding can enhance photocatalytic H2 evolution efficiency. J. Mater. Chem. A 2013, 1, 14089–14096. [Google Scholar] [CrossRef]
- Li, Y.; Ouyang, S.; Xu, H.; Wang, X.; Bi, Y.; Zhang, Y.; Ye, J. Constructing Solid-Gas-Interfacial Fenton Reaction over Alkalinized-C3N4 Photocatalyst to Achieve Apparent Quantum Yield of 49% at 420 nm. J. Am. Chem. Soc. 2016, 138, 13289–13297. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Azam, Q.; Mohsin, J.; Sammia, S.; Mudassar, S. Fabrication of g-C3N4/transition metal (Fe, Co, Ni, Mn and Cr)-doped ZnO ternary composites: Excellent visible light active photocatalysts for the degradation of organic pollutants from wastewater. Mater. Res. Bull. 2021, 147, 111630. [Google Scholar]
- Chen, P.-W.; Li, K.; Yu, Y.-X.; Zhang, W.-D. Cobalt-doped graphitic carbon nitride photocatalysts with high activity for hydrogen evolution. Appl. Surf. Sci. 2017, 392, 608–615. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, Y.; Wang, L.; Ai, S.; Ding, H. Cu-modified alkalinized g-C3N4 as photocatalytically assisted heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2017, 426, 1133–1140. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Huang, S.; Li, Y.; Zhang, X.; Zeng, G.; Niu, L.; Ling, Y.; Zhang, Y. Facile construction of 2D g-C3N4 supported nanoflower-like NaBiO3 with direct Z-scheme heterojunctions and insight into its photocatalytic degradation of tetracycline. J. Hazard. Mater. 2021, 414, 125547. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Ou, M.; Zhong, Q.; Zhang, S.; Yu, L. Efficient visible-light photocatalytic oxidation of gaseous NO with graphitic carbon nitride (g-C3N4) activated by the alkaline hydrothermal treatment and mechanism analysis. J. Hazard. Mater. 2015, 300, 598–606. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zhao, W.L.; Chen, Y. g-C3N4 modified by hydroxyl group on the surface prepared by double salt enhanced the visible light photocatalytic activity. Diam. Relat. Mater. 2021, 116, 108425. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, Z.; Deng, S.; Wang, H.; Wu, Z. Decorating g-C3N4 with alkalinized Ti3C2 Mxene for promoted photocatalytic CO2 reduction performance. J. Colloid. Interface Sci. 2020, 564, 406–417. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Lu, G. Highly Efficient Hydrogen Evolution over Co(OH)2 Nanoparticles Modified g-C3N4 Co-sensitized by Eosin Y and Rose Bengal under Visible Light Irradiation. Appl. Catal. B Environ. 2016, 188, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, J. Graphene supported Co-g-C3N4 as a novel metal-macrocyclic electrocatalyst for the oxygen reduction reaction in fuel cells. Langmuir 2013, 29, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Kong, L.; Dong, Y.; Wang, G.; Wu, X.; Jiang, P. Insight into the crucial factors for photochemical deposition of cobalt cocatalysts on g-C3N4 Photocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 9522–9531. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Qin, F.; Jiang, Y.; An, H.; Fang, J.; Zhang, K.; Lai, Y. Mesoporous Co–N–C composite as a sulfur host for high-capacity and long-life lithium–sulfur batteries. J. Mater. Sci. 2018, 53, 13143–13155. [Google Scholar] [CrossRef]
- Yu, J.; Chen, G.; Sunarso, J.; Zhu, Y.; Ran, R.; Zhu, Z.; Zhou, W.; Shao, Z. Cobalt oxide and cobalt-graphitic carbon core-shell based catalysts with remarkably high oxygen reduction reaction activity. Adv. Sci. 2016, 3, 1600060. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, D.; Zhou, Y.; Qin, F.; Wang, H.; Wang, W.; Yang, Y.; Zeng, G. Dual optimization approach to Mo single atom dispersed g-C3N4 photocatalyst: Morphology and defect evolution. Appl. Catal. B Environ. 2022, 303, 120904. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Li, J.; Gao, G.; Zhi, J. Onion-liked carbon-embedded graphitic carbon nitride for enhanced photocatalytic hydrogen evolution and dye degradation. Appl. Catal. B: Environ. 2022, 308, 121216. [Google Scholar] [CrossRef]
- Chen, T.; Yin, D.; Zhao, F.; Kyu, K.K.; Liu, B.; Chen, D.; Huang, K.; Deng, L.; Li, L. Fabrication of 2D heterojunction photocatalyst Co-g-C3N4/MoS2 with enhanced solar-light-driven photocatalytic activity. New J. Chem. 2019, 43, 463–473. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, Z.; Huang, D.; Qin, H.; He, Y.; Chen, M.; Zeng, G.; Xu, P. Ferrocene modified g-C3N4 as a heterogeneous catalyst for photo-assisted activation of persulfate for the degradation of tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127024. [Google Scholar] [CrossRef]
- Yang, G.; Liang, Y.J.; Xiong, Z.R.; Yang, J.; Wang, K.; Zeng, Z.K. Molten salt-assisted synthesis of Ce4O7/Bi4MoO9 heterojunction photocatalysts for photo-Fenton degradation of tetracycline: Enhanced mechanism, degradation pathway and products toxicity assessment. Chem. Eng. J. 2021, 425, 130689. [Google Scholar] [CrossRef]
- Shi, C.D.; Yu, S.Y.; Li, C.J. Fabrication of aligned carbon nanofiber doped with SnO2-Sb for efficient electrochemical removal of tetracycline. Chem. Eng. J. 2022, 441, 136052. [Google Scholar] [CrossRef]
- Duan, R.; Ma, S.; Xu, S.; Wang, B.; He, M.; Li, G.; Fu, H.; Zhao, P. Soybean straw biochar activating peroxydisulfate to simultaneously eliminate tetracycline and tetracycline resistance bacteria: Insights on the mechanism. Water Res. 2022, 218, 118489. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Guo, F.; Shi, Y.; Li, L.; Xu, Z.; Yan, X.; Shi, W. Fe-doped g-C3N4 derived from biowaste material with Fe-N bonds for enhanced synergistic effect between photocatalysis and fenton degradation activity in a broad pH range. J. Alloy. Compd. 2022, 900, 163410. [Google Scholar] [CrossRef]
- Bai, X.; Wang, X.; Lu, X.; Liang, Y.; Li, J.; Wu, L.; Li, H.; Hao, Q.; Ni, B.-J.; Wang, C. Surface defective g-C3N4-xClx with unique spongy structure by polarization effect for enhanced photocatalytic removal of organic pollutants. J. Hazard. Mater. 2020, 398, 122897. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Photo-accelerated Co3+/Co2+ transformation on cobalt and phosphorus Co-doped g-C3N4 for Fenton-like reaction. J. Mater. Chem. A 2021, 9, 22399–22409. [Google Scholar] [CrossRef]













| Sample | SBET (m2g−1) | Pore Volume (cm3g−1) | Average Pore Size (nm) |
|---|---|---|---|
| g-C3N4 | 15.279 | 0.066 | 3.924 |
| CNK-OH | 5.416 | 0.019 | 3.130 |
| 9%Co-CNK-OH | 8.243 | 0.033 | 3.926 |
| Sample | Eg (eV) | EFB (V vs. Ag/AgCl) | ECB (V vs. NHE) | EVB (V) |
|---|---|---|---|---|
| g-C3N4 | 2.71 | −0.437 | −0.24 | 2.47 |
| CNK-OH | 2.69 | −0.458 | −0.261 | 2.429 |
| 3%Co-CNK-OH | 2.72 | −0.486 | −0.289 | 2.231 |
| 6%Co-CNK-OH | 2.71 | −0.433 | −0.236 | 2.474 |
| 9%Co-CNK-OH | 2.68 | −0.487 | −0.29 | 2.39 |
| 12%Co-CNK-OH | 2.69 | −0.427 | −0.23 | 2.46 |
| 15%Co-CNK-OH | 2.69 | −0.374 | −0.187 | 2.503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, D.; Luo, J.; Sun, Q.; Zhang, M.; Wang, J. Preparation of Co-CNK-OH and Its Performance in Fenton-like Photocatalytic Degradation of Tetracycline. Catalysts 2023, 13, 715. https://doi.org/10.3390/catal13040715
Hou D, Luo J, Sun Q, Zhang M, Wang J. Preparation of Co-CNK-OH and Its Performance in Fenton-like Photocatalytic Degradation of Tetracycline. Catalysts. 2023; 13(4):715. https://doi.org/10.3390/catal13040715
Chicago/Turabian StyleHou, Dongrui, Jing Luo, Qinggong Sun, Mengyang Zhang, and Jianfeng Wang. 2023. "Preparation of Co-CNK-OH and Its Performance in Fenton-like Photocatalytic Degradation of Tetracycline" Catalysts 13, no. 4: 715. https://doi.org/10.3390/catal13040715
APA StyleHou, D., Luo, J., Sun, Q., Zhang, M., & Wang, J. (2023). Preparation of Co-CNK-OH and Its Performance in Fenton-like Photocatalytic Degradation of Tetracycline. Catalysts, 13(4), 715. https://doi.org/10.3390/catal13040715