Nano-Magnetic CaO/Fe2O3/Feldspar Catalysts for the Production of Biodiesel from Waste Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CaO/Fe2O3 and CaO/Fe2O3/feldspar Nano-Magnetic Catalyst
2.1.1. XRD Analysis
2.1.2. FESEM and EDX Analysis
2.1.3. Porosity and Surface Area Analysis
2.1.4. VSM Analysis
2.2. Optimization of Transesterification Reaction Process Parameters
2.3. Proposed Mechanism of CaO/Fe2O3/Feldspar Catalyst for Transesterification
2.4. Comparison of Catalytic Activity with Published Literature Reported on Magnetic Catalysts
2.5. CaO/Fe2O3/Feldspar Catalyst Reusability for Karanja, Wild Mustard, and Safflower Seed Oil
2.6. Physicochemical Properties
2.7. Fatty Acid Profile
3. Materials and Methods
3.1. Materials and Chemical Reagent
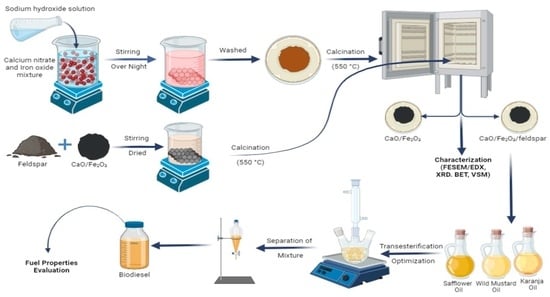
3.2. Preparation of Supported Nano-Magnetic Catalyst
3.3. Characterization of Catalyst and Esters
3.4. Transesterification and Physiochemical Properties Evaluation
3.5. CaO/Fe2O3/Feldspar Catalyst Reusability Test
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Ali, A.; Mushtaq, A. Do biofuels really a need of the day: Approaches, mechanisms, applications and challenges. Int. J. Chem. Biochem. Sci. 2023, 23, 219–226. [Google Scholar]
- Sanjid, A.; Masjuki, H.; Kalam, M.; Abedin, M.; Rahman, S.A. Experimental investigation of mustard biodiesel blend properties, performance, exhaust emission and noise in an unmodified diesel engine. APCBEE Procedia 2014, 10, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Siddique, H.S.; Nadeem, F.; Inam, S.; Kazerooni, E.A. Recent production methodologies and advanced spectroscopic characterization of biodiesel: A review. Int. J. Chem. Biochem. Sci. 2020, 18, 134–144. [Google Scholar]
- Lokman, I.M.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Carbohydrate-derived solid acid catalysts for biodiesel production from low-cost feedstocks: A review. Catal. Rev. Sci. Eng. 2014, 56, 187–219. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Haj, L.; Al-Muhtaseb, A.H.; Al-Hinai, M.A.; Baawain, M.; Rashid, U.; Ahmad, M.N.M. Current scenario of catalysts for biodiesel production: A critical review. Rev. Chem. Eng. 2018, 34, 267–297. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Gupta, V.K.; Manikanta, A.; Mishra, K.; Singh, S.; Singh, S.; Ramteke, P.; Mishra, P. Recent development on sustainable biodiesel production using sewage sludge. 3 Biotech 2018, 8, 245. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Aslam, K.; Mushtaq, A.; Nadeem, F.; Ghnia, J.B.; Rafique, M.; Sillanpää, M. Economic feasibility of non-edible oils as biodiesel feedstock: A brief. Int. J. Chem. Biochem. Sci. 2020, 18, 145–150. [Google Scholar]
- Irshad, F.; Mushtaq, A. Biomass-derived Materials and their Commercial Applications. Int. J. Chem. Biochem. Sci. 2020, 23, 202–211. [Google Scholar]
- Ikram, M.M.; Hanif, M.A.; Khan, G.S.; Rashid, U.; Nadeem, F. Significant seed oil feedstocks for renewable production of biodiesel: A review. Curr. Org. Chem. 2019, 23, 1509–1516. [Google Scholar] [CrossRef]
- Xie, W.; Li, J. Magnetic solid catalysts for sustainable and cleaner biodiesel production: A comprehensive review. Renews 2023, 171, 113017. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Basic ionic liquid functionalized magnetically responsive Fe3O4@HKUST-1 composites used for biodiesel production. Fuel 2018, 220, 248–256. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Grafting copolymerization of dual acidic ionic liquid on core-shell structured magnetic silica: A magnetically recyclable Brönsted acid catalyst for biodiesel production by one-pot transformation of low-quality oils. Fuel 2021, 283, 118893. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Rashid, U. Supported solid and heteropoly acid catalysts for production of biodiesel. Catal. Rev. 2017, 59, 165–188. [Google Scholar] [CrossRef]
- Xue, B.; Guo, H.; Liu, L.; Chen, M. Preparation, characterization and catalytic properties of yttrium-zirconium-pillared montmorillonite and their application in supported Ce catalysts. Clay Miner. 2015, 50, 211–219. [Google Scholar] [CrossRef]
- Keshavarz, M.; Papari, R.F.; Bulgariu, L.; Esmaeili, H. Synthesis of CaO/Fe2O3 nanocomposite as an efficient nanoadsorbent for the treatment of wastewater containing Cr (III). Sep. Sci. Technol. 2021, 56, 1328–1341. [Google Scholar] [CrossRef]
- Ali, B.J.; Othman, S.S.; Harun, F.W.; Jumal, J.; Rahman, M.B.A. Immobilization of enzyme using natural feldspar for use in the synthesis of oleyl oleate. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; p. 030018. [Google Scholar]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H. Production of biodiesel from palm fatty acid distillate using sulfonated-glucose solid acid catalyst: Characterization and optimization. Chin. J. Chem. Eng. 2015, 23, 1857–1864. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Pristiyani, R.; Dewajani, H. A new route of biodiesel production through chemical interesterification of jatropha oil using ethyl acetate. Int. J. ChemTech Res. 2016, 9, 627–634. [Google Scholar]
- Hazmi, B.; Rashid, U.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Nehdi, I.A. Supermagnetic nano-bifunctional catalyst from rice husk: Synthesis, characterization and application for conversion of used cooking oil to biodiesel. Catalysts 2020, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.A.; Rashid, U.; Hazmi, B.; Moser, B.R.; Alharthi, F.A.; Rokhum, S.L.; Ngamcharussrivichai, C. Biodiesel production from waste cooking oil using magnetic bifunctional calcium and iron oxide nanocatalysts derived from empty fruit bunch. Fuel 2022, 317, 123525. [Google Scholar] [CrossRef]
- Xie, W.; Han, Y.; Wang, H. Magnetic Fe3O4/MCM-41 composite-supported sodium silicate as heterogeneous catalysts for biodiesel production. Renew Energy 2018, 125, 675–681. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Pua, F.; Syed Jaafar, S.N. Synthesis and characterization of local biomass supported magnetic catalyst for esterification reaction. Mater. Today Proc. 2020, 31, 161–165. [Google Scholar] [CrossRef]
- Ali, R.M.; Elkatory, M.R.; Hamad, H.A. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 2020, 268, 117297. [Google Scholar] [CrossRef]
- Saeed, A.; Hanif, M.A.; Hanif, A.; Rashid, U.; Iqbal, J.; Majeed, M.I.; Moser, B.R.; Alsalme, A. production of biodiesel from Spirogyra elongata, a common freshwater green algae with high oil content. Sustainability 2021, 13, 12737. [Google Scholar] [CrossRef]
- Ijaz, B.; Hanif, M.A.; Rashid, U.; Zubair, M.; Mushtaq, Z.; Nawaz, H.N.; Choong, T.S.Y.; Nehdi, I.A. High vacuum fractional distillation (HVFD) approach for quality and performance improvement of Azadirachta indica biodiesel. Energies 2020, 13, 2858. [Google Scholar] [CrossRef]
- Ismail, S.A.-E.A.; Ali, R.F.M. Physico-chemical properties of biodiesel manufactured from waste frying oil using domestic adsorbents. Science and technology of advanced materials. Sci. Technol. Adv. Mater. 2015, 16, 034602. [Google Scholar] [CrossRef] [Green Version]
- Folayan, A.J.; Anawe, P.A.L.; Aladejare, A.E.; Ayeni, A.O. Experimental investigation of the effect of fatty acids configuration, chain length, branching and degree of unsaturation on biodiesel fuel properties obtained from lauric oils, high-oleic and high-linoleic vegetable oil biomass. Energy Rep. 2019, 5, 793–806. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Shameer, P.M. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Islam, A.K.M.A.; Chakrabarty, S.; Yaakob, Z.; Ahiduzzaman, M.; Islam, A.K.M.M. Koroch (Pongamia pinnata): A promising unexploited resources for the tropics and subtropics. In Forest Biomass-From Trees to Energy; IntechOpen: London, UK, 2021. [Google Scholar]
- Hanif, M.; Bhatti, H.N.; Hanif, M.A.; Rashid, U.; Hanif, A.; Moser, B.R.; Alsalme, A. A novel heterogeneous superoxide support-coated catalyst for production of biodiesel from roasted and unroasted Sinapis arvensis seed oil. Catalysts 2021, 11, 1421. [Google Scholar] [CrossRef]
- Murthy, I.; Anjani, K. Fatty acid composition in Carthamus species. In Proceedings of the 7th International Safflower Conference, Wagga Wagga, Australia, 3–6 November 2008; Australian Oilseeds Federation: Sydney, Australia, 2007. [Google Scholar]
- Ali, M.A.; Al-Hydary, I.A.; Al-Hattab, T.A. Nano-magnetic catalyst CaO-Fe3O4 for biodiesel production from date palm seed oil. Bull. Chem. React. Eng. Catal. 2017, 12, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Zhang, P.; Fan, M.; Jiang, P.; Dong, Y. Influence of crystal of Fe2O3 in magnetism and activity of nanoparticle CaO@ Fe2O3 for biodiesel production. Fuel 2017, 197, 343–347. [Google Scholar] [CrossRef]






| Catalyst | Element (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| O | Na | Al | Si | K | Ca | Fe | Total | |
| CaO/Fe2O3 | 37.12 | - | - | - | - | 3.06 | 59.82 | 100 |
| CaO/Fe2O3/feldspar | 43.46 | 0.99 | 2.18 | 6.69 | 1.34 | 8.66 | 36.68 | 100 |
| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| CaO/Fe2O3 | 68.680 | 0.189 | 7.670 |
| CaO/Fe2O3/feldspar | 19.523 | 0.060 | 7.911 |
| Feedstock | Catalyst Conc. (%) | Methanol-to-Oil Ratio | Biodiesel Yield (%) |
|---|---|---|---|
| Karanja | 0.5 | 5:1 | 85.0 ± 0.5 |
| 1 | 5:1 | 89.1 ± 0.8 | |
| 1.5 | 5:1 | 87.5 ± 0.7 | |
| 2 | 5:1 | 86.0 ± 0.6 | |
| 2.5 | 5:1 | 85.6 ± 0.5 | |
| 1 | 10:1 | 99.9 ± 0.9 | |
| 1 | 15:1 | 88.1 ± 0.7 | |
| 1 | 20:1 | 85.6 ± 0.8 | |
| 1 | 25:1 | 83.7 ± 0.5 | |
| Wild mustard | 0.5 | 5:1 | 85.0 ± 0.7 |
| 1 | 5:1 | 93.6 ± 0.3 | |
| 1.5 | 5:1 | 84.7 ± 0.9 | |
| 2 | 5:1 | 82.9 ± 0.5 | |
| 2.5 | 5:1 | 81.8 ± 0.8 | |
| 1 | 10:1 | 85.4 ± 0.6 | |
| 1 | 15:1 | 83.2 ± 0.3 | |
| 1 | 20:1 | 80.5 ± 0.9 | |
| 1 | 25:1 | 79.8 ± 0.4 | |
| Wild safflower | 0.5 | 5:1 | 85.0 ± 0.5 |
| 1 | 5:1 | 85.9 ± 0.7 | |
| 1.5 | 5:1 | 99.3 ± 0.7 | |
| 2 | 5:1 | 86.5 ± 0.5 | |
| 2.5 | 5:1 | 85.0 ± 0.6 | |
| 1.5 | 10:1 | 86.0 ± 0.3 | |
| 1.5 | 15:1 | 84.5 ± 0.9 | |
| 1.5 | 20:1 | 82.3 ± 0.8 | |
| 1.5 | 25:1 | 80.4 ± 0.6 |
| Types of Catalyst | Experimental Reaction Conditions | Esters Yield (%) | References |
|---|---|---|---|
| RHC/K2O-20%/Fe-5% | Methanol-to-oil ratio = 12:1, temp. = 75 °C, time = 4 h, catalyst loading = 4 wt.% | 98.60 | [21] |
| CaO-Fe2O3/AC | Methanol-to-oil ratio = 18:1, temp. = 65 °C, time = 3 h, catalyst loading = 3 wt.% | 98.30 | [22] |
| Fe3O4/MCM-41 composites | Methanol-to-oil ratio = 25:1, time = 8 h, catalyst loading = 3 wt.% | 99.20 | [23] |
| EFB supported magnetic solid acid catalyst | Catalyst loading = 10 wt.% | 87.32 | [24] |
| Magnetically recyclable CuFe2O4 | Methanol-to-oil ratio = 18:1, temp. = 60 °C, time = 0.5 h, catalyst loading = 3 wt.% | 90.24 | [25] |
| CaO/Fe2O3/feldspar | Methanol-to-oil ratio = 10:1, catalyst loading = 1 wt.% (karanja oil) Methanol-to-oil ratio = 5:1, catalyst loading = 1 wt.% (wild mustard oil) Methanol-to-oil ratio = 5:1, catalyst loading = 1.5 wt.% (safflower oil) | 99.9 93.6 99.3 | Present study |
| Fuel Parameters | Karanja | Wild Mustard | Wild Safflower | ASTM D6751 Limits |
|---|---|---|---|---|
| Density (g/mL) | 0.85 | 0.86 | 0.88 | Not specified |
| Cloud point (°C) | 4.0 | 2.1 | 0.8 | Report |
| Pour point (°C) | −1.1 | −1.6 | −3.9 | Not specified |
| Acid value (mg KOH/g) | 0.15 | 0.46 | 0.15 | 0.50 max |
| Iodine value (g I2/100 g) | 82.5 | 86.65 | 69.44 | Not specified |
| Saponification value (mg KOH g–1 oil) | 180.04 | 175.04 | 196.58 | Not specified |
| Cetene number | 58.05 | 57.98 | 58.44 | 47 minimum |
| Fatty Acid | Molecular Formula | Fatty Acid Amount (%) | ||
|---|---|---|---|---|
| Karanja Oil | Wild Mustard Oil | Wild Safflower Oil | ||
| Capric acid | C10H20O2 | 0.11 | 0.15 | 0.13 |
| Lauric acid | C12H24O2 | 0.22 | 0.12 | 0.09 |
| Myristic acid | C14H28O2 | 0.93 | 0.18 | 0.16 |
| Palmitic acid | C16H32O2 | 10.33 | 3.63 | 7.73 |
| Linolenic acid | C18H30O2 | 3.15 | 0.09 | 0.32 |
| Linoleic acid | C18H32O2 | 11.03 | 15.75 | 75.17 |
| Oleic acid | C18H34O2 | 51.92 | 23.11 | 12.98 |
| Stearic acid | C18H36O2 | 4.66 | 1.15 | 0.89 |
| Eicosanoic acid | C20H40O2 | 9.76 | 12.83 | 0.11 |
| Erucic acid | C22H42O2 | - | 41.43 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, M.; Bhatti, I.A.; Hanif, M.A.; Rashid, U.; Moser, B.R.; Hanif, A.; Alharthi, F.A. Nano-Magnetic CaO/Fe2O3/Feldspar Catalysts for the Production of Biodiesel from Waste Oils. Catalysts 2023, 13, 998. https://doi.org/10.3390/catal13060998
Hanif M, Bhatti IA, Hanif MA, Rashid U, Moser BR, Hanif A, Alharthi FA. Nano-Magnetic CaO/Fe2O3/Feldspar Catalysts for the Production of Biodiesel from Waste Oils. Catalysts. 2023; 13(6):998. https://doi.org/10.3390/catal13060998
Chicago/Turabian StyleHanif, Maryam, Ijaz Ahmad Bhatti, Muhammad Asif Hanif, Umer Rashid, Bryan R. Moser, Asma Hanif, and Fahad A. Alharthi. 2023. "Nano-Magnetic CaO/Fe2O3/Feldspar Catalysts for the Production of Biodiesel from Waste Oils" Catalysts 13, no. 6: 998. https://doi.org/10.3390/catal13060998
APA StyleHanif, M., Bhatti, I. A., Hanif, M. A., Rashid, U., Moser, B. R., Hanif, A., & Alharthi, F. A. (2023). Nano-Magnetic CaO/Fe2O3/Feldspar Catalysts for the Production of Biodiesel from Waste Oils. Catalysts, 13(6), 998. https://doi.org/10.3390/catal13060998