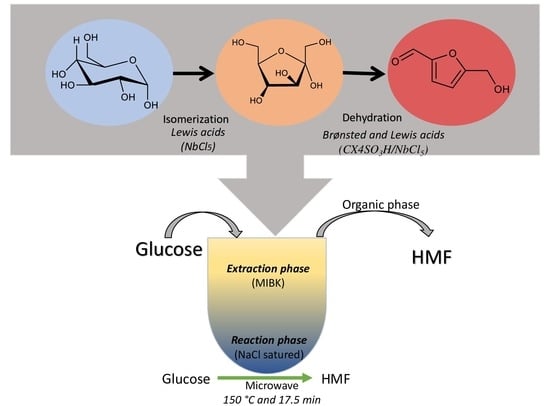
Conversion of Glucose to 5-Hydroxymethylfurfural Using Consortium Catalyst in a Biphasic System and Mechanistic Insights
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Different Niobium Catalysts in HMF Synthesis
2.2. Evaluation of CX4SO3H/NbCl5 Consortium Catalyst Ratio
2.3. Evaluation of Temperature and Time
2.4. Evaluation of the Addition of Different Salt in Biphasic System
2.5. Evaluation of Catalytic Effect of Other Lewis Acids
2.6. Literature Comparison of Methods for the Conversion Glucose to HMF
2.7. Evaluation of Other Carbohydrates to Produce HMF
2.8. Catalyst Recycling
2.9. Reaction Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CX4SO3H
3.3. General Procedure for Conversion of Glucose into HMF
3.4. Quantification of HMF by UHPLC
3.5. Catalyst Recycling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yin, Y.; Ma, C.; Li, W.; Luo, S.; Liu, Y.; Wu, X.; Wu, Z.; Liu, S. Rapid Conversion of Glucose to 5-Hydroxymethylfurfural Using a MoCl3 Catalyst in an Ionic Liquid with Microwave Irradiation. Ind. Crops Prod. 2021, 160, 113091. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y.; Wang, Y.; Dai, L. Enhanced Conversion of α-Cellulose to 5-HMF in Aqueous Biphasic System Catalyzed by FeCl3-CuCl2. Chinese Chem. Lett. 2021, 32, 2233–2238. [Google Scholar] [CrossRef]
- Kong, Q.S.; Li, X.L.; Xu, H.J.; Fu, Y. Conversion of 5-Hydroxymethylfurfural to Chemicals: A Review of Catalytic Routes and Product Applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Pereira, S.; Oliveira Santana Varejão, J.; de Fátima, Â.; Fernandes, S.A. P-Sulfonic Acid Calix[4]Arene: A Highly Efficient Organocatalyst for Dehydration of Fructose to 5-Hydroxymethylfurfural. Ind. Crops Prod. 2019, 138, 4–10. [Google Scholar] [CrossRef]
- El Fergani, M.; Candu, N.; Tudorache, M.; Bucur, C.; Djelal, N.; Granger, P.; Coman, S.M. From Useless Humins By-Product to Nb@graphite-like Carbon Catalysts Highly Efficient in HMF Synthesis. Appl. Catal. A Gen. 2021, 618, 118130. [Google Scholar] [CrossRef]
- Torres-Olea, B.; García-Sancho, C.; Cecilia, J.A.; Oregui-Bengoechea, M.; Arias, P.L.; Moreno-Tost, R.; Maireles-Torres, P. Influence of Lewis Acidity and CaCl2 on the Direct Transformation of Glucose to 5-Hydroxymethylfurfural. Mol. Catal. 2021, 510, 111685. [Google Scholar] [CrossRef]
- Vieira, J.L.; Almeida-Trapp, M.; Mithöfer, A.; Plass, W.; Gallo, J.M.R. Rationalizing the Conversion of Glucose and Xylose Catalyzed by a Combination of Lewis and Brønsted Acids. Catal. Today 2020, 344, 92–101. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, J.; Yagoub, A.E.G.A.; Ma, H.; Yu, X.; Hu, J.; Bao, X.; Liu, S. Conversion of Glucose into 5-Hydroxymethylfurfural in Different Solvents and Catalysts: Reaction Kinetics and Mechanism. Egypt. J. Pet. 2017, 26, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ding, J.; Jiang, J.G.; Zhu, Z.; Wu, P. One-Pot Synthesis of 5-Hydroxymethylfurfural from Glucose Using Bifunctional [Sn,Al]-Beta Catalysts. Cuihua Xuebao/Chinese J. Catal. 2015, 36, 820–828. [Google Scholar] [CrossRef]
- Teimouri, A.; Mazaheri, M.; Chermahini, A.N.; Salavati, H.; Momenbeik, F.; Fazel-Najafabadi, M. Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural (HMF) Using Nano-POM/Nano-ZrO2/Nano-γ-Al2O3. J. Taiwan Inst. Chem. Eng. 2015, 49, 40–50. [Google Scholar] [CrossRef]
- Wang, T.; Glasper, J.A.; Shanks, B.H. Kinetics of Glucose Dehydration Catalyzed by Homogeneous Lewis Acidic Metal Salts in Water. Appl. Catal. A Gen. 2015, 498, 214–221. [Google Scholar] [CrossRef]
- Abranches, P.A.D.S.; De Paiva, W.F.; De Fátima, Â.; Martins, F.T.; Fernandes, S.A. Calix[n]Arene-Catalyzed Three-Component Povarov Reaction: Microwave-Assisted Synthesis of Julolidines and Mechanistic Insights. J. Org. Chem. 2018, 83, 1761–1771. [Google Scholar] [CrossRef]
- Braga, I.B.; Castañeda, S.M.B.; Vitor De Assis, J.; Barros, A.O.; Amarante, G.W.; Valdo, A.K.S.M.; Martins, F.T.; Rosolen, A.F.D.P.; Pilau, E.; Fernandes, S.A. Anise Essential Oil as a Sustainable Substrate in the Multicomponent Double Povarov Reaction for Julolidine Synthesis. J. Org. Chem. 2020, 85, 15622–15630. [Google Scholar] [CrossRef] [PubMed]
- Liberto, N.A.; Simões, J.B.; de Paiva Silva, S.; da Silva, C.J.; Modolo, L.V.; de Fátima, Â.; Silva, L.M.; Derita, M.; Zacchino, S.; Zuñiga, O.M.P.; et al. Quinolines: Microwave-Assisted Synthesis and Their Antifungal, Anticancer and Radical Scavenger Properties. Bioorganic Med. Chem. 2017, 25, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Natalino, R.; Varejão, E.V.V.; da Silva, M.J.; Cardoso, A.L.; Fernandes, S.A. P-Sulfonic Acid Calix[n]Arenes: The Most Active and Water Tolerant Organocatalysts in Esterification Reactions. Catal. Sci. Technol. 2014, 4, 1369–1375. [Google Scholar] [CrossRef]
- Palermo, V.; Sathicq, A.; Liberto, N.; Fernandes, S.; Langer, P.; Jios, J.; Romanelli, G. Calix[n]Arenes: Active Organocatalysts for the Synthesis of Densely Functionalized Piperidines by One-Pot Multicomponent Procedure. Tetrahedron Lett. 2016, 57, 2049–2054. [Google Scholar] [CrossRef]
- Rezende, T.R.M.; Varejão, J.O.S.; Sousa, A.L.L.D.A.; Castañeda, S.M.B.; Fernandes, S.A. Tetrahydroquinolines by the Multicomponent Povarov Reaction in Water: Calix[: N] Arene-Catalysed Cascade Process and Mechanistic Insights. Org. Biomol. Chem. 2019, 17, 2913–2922. [Google Scholar] [CrossRef]
- Santos, L.S.; Fernandes, S.A.; Pilli, R.A.; Marsaioli, A.J. A Novel Asymmetric Reduction of Dihydro-β-Carboline Derivatives Using Calix[6]Arene/Chiral Amine as a Host Complex. Tetrahedron Asymmetry 2003, 14, 2515–2519. [Google Scholar] [CrossRef]
- Simões, J.B.; De Fátima, Â.; Sabino, A.A.; De Aquino, F.J.T.; Da Silva, D.L.; Barbosa, L.C.A.; Fernandes, S.A. Organocatalysis in the Three-Component Povarov Reaction and Investigation by Mass Spectrometry. Org. Biomol. Chem. 2013, 11, 5069–5073. [Google Scholar] [CrossRef]
- Simões, J.B.; De Fátima, Â.; Sabino, A.A.; Almeida Barbosa, L.C.; Fernandes, S.A. Efficient Synthesis of 2,4-Disubstituted Quinolines: Calix[n]Arene- Catalyzed Povarov-Hydrogen-Transfer Reaction Cascade. RSC Adv. 2014, 4, 18612–18615. [Google Scholar] [CrossRef]
- David, G.F.; Ríos-Ríos, A.M.; de Fátima, Â.; Perez, V.H.; Fernandes, S.A. The Use of P-Sulfonic Acid Calix[4]Arene as Organocatalyst for Pretreatment of Sugarcane Bagasse Increased the Production of Levoglucosan. Ind. Crops Prod. 2019, 134, 382–387. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I. The Role of Niobium Component in Heterogeneous Catalysts. Catal. Today 2017, 285, 211–225. [Google Scholar] [CrossRef]
- David, G.F.; Pereira, S.d.P.S.; Fernandes, S.A.; Cubides-Roman, D.C.; Siqueira-Roman, R.K.; Perez, V.H.; Lacerda, V. Fast Pyrolysis as a Tool for Obtaining Levoglucosan after Pretreatment of Biomass with Niobium Catalysts. Waste Manag. 2021, 126, 274–282. [Google Scholar] [CrossRef]
- Junior, M.M.d.J.; Fernandes, S.A.; Borges, E.; Baêta, B.E.L.; Rodrigues, F.d.Á. Kinetic Study of the Conversion of Glucose to 5-Hydroxymethylfurfural Using Niobium Phosphate. Mol. Catal. 2022, 518, 112079. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Bossola, F.; Dal Santo, V. Cooperative Action of Brønsted and Lewis Acid Sites of Niobium Phosphate Catalysts for Cellobiose Conversion in Water. Appl. Catal. B Environ. 2016, 193, 93–102. [Google Scholar] [CrossRef]
- Catrinck, M.N.; Ribeiro, E.S.; Monteiro, R.S.; Ribas, R.M.; Barbosa, M.H.P.; Teófilo, R.F. Direct Conversion of Glucose to 5-Hydroxymethylfurfural Using a Mixture of Niobic Acid and Niobium Phosphate as a Solid Acid Catalyst. Fuel 2017, 210, 67–74. [Google Scholar] [CrossRef]
- Morales, A.; Santana, A.; Althoff, G.; Melendez, E. Host-Guest Interactions between Calixarenes and Cp2NbCl 2. J. Organomet. Chem. 2011, 696, 2519–2527. [Google Scholar] [CrossRef] [Green Version]
- Redshaw, C.; Rowan, M.; Homden, D.M.; Elsegood, M.R.J.; Yamato, T.; Pérez-Casas, C. Niobium- and Tantalum-Based Ethylene Polymerisation Catalysts Bearing Methylene- or Dimethyleneoxa-Bridged Calixarene Ligands. Chem.-A Eur. J. 2007, 13, 10129–10139. [Google Scholar] [CrossRef] [PubMed]
- Acho, J.A.; Doerrer, L.H.; Lippard, S.J. Pentamethylcyclopentadienyl and Cyclopentadienyl Tantalum and Niobium Calixarene Compounds and Their Water and Acetonitrile Inclusion Complexes. Inorg. Chem. 1995, 34, 2542–2556. [Google Scholar] [CrossRef]
- Dürr, S.; Bechlars, B.; Radius, U. Calix[4]Arene Supported Group 5 Imido Complexes. Inorganica Chim. Acta 2006, 359, 4215–4226. [Google Scholar] [CrossRef]
- Caselli, A.; Solari, E.; Scopelliti, R.; Floriani, C. A Synthetic Methodology to Niobium Alkylidenes: Reactivity of a Nb=Nb Double Bond Anchored to a Calix[4]Arene Oxo Surface with Ketones, Aldehydes, Imines, and Isocyanides. J. Am. Chem. Soc. 1999, 121, 8296–8305. [Google Scholar] [CrossRef]
- Caselli, A.; Solari, E.; Scopelliti, R.; Floriani, C.; Re, N.; Rizzoli, C.; Chiesi-Villa, A. Dinitrogen Rearranging over a Metal-Oxo Surface and Cleaving to Nitride: From the End-on to the Side-on Bonding Mode, to the Stepwise Cleavage of the N≡N Bonds Assisted by Nb(III)-Calix[4]Arene. J. Am. Chem. Soc. 2000, 122, 3652–3670. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Biella, S.; Auroux, A. Niobic Acid and Niobium Phosphate as Highly Acidic Viable Catalysts in Aqueous Medium: Fructose Dehydration Reaction. Catal. Today 2006, 118, 373–378. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Zhu, X. Study on Two-Step Pyrolysis of Soybean Stalk by TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2017, 127, 91–98. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Nakagawa, K.; Peng, Y.K.; Koito, Y.; Zheng, J.; Tsang, S.C.E. Niobium Oxides: Correlation of Acidity with Structure and Catalytic Performance in Sucrose Conversion to 5-Hydroxymethylfurfural. J. Catal. 2016, 338, 329–339. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; He, J.; Zhao, W.; Yang, T.; Yang, S. Catalytic Conversion of Carbohydrates to Levulinic Acid with Mesoporous Niobium-Containing Oxides. Catal. Commun. 2017, 93, 20–24. [Google Scholar] [CrossRef]
- Huang, F.; Jiang, T.; Dai, H.; Xu, X.; Jiang, S.; Chen, L.; Fei, Z.; Dyson, P.J. Transformation of Glucose to 5-Hydroxymethylfurfural Over Regenerated Cellulose Supported Nb2O5·nH2O in Aqueous Solution. Catal. Letters 2020, 150, 2599–2606. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, M.; Xu, H.; Wang, Y.; Yan, K. Recent Advance on the Catalytic System for Efficient Production of Biomass-Derived 5-Hydroxymethylfurfural. Renew. Sustain. Energy Rev. 2021, 147. [Google Scholar] [CrossRef]
- Kim, Y.; Mittal, A.; Robichaud, D.J.; Pilath, H.M.; Etz, B.D.; St. John, P.C.; Johnson, D.K.; Kim, S. Prediction of Hydroxymethylfurfural Yield in Glucose Conversion through Investigation of Lewis Acid and Organic Solvent Effects. ACS Catal. 2020, 10, 14707–14721. [Google Scholar] [CrossRef]
- Swift, T.D.; Nguyen, H.; Erdman, Z.; Kruger, J.S.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis Acid/Brønsted Acid-Catalyzed Conversion of Carbohydrates to 5-Hydroxymethylfurfural Using Zeolite Beta. J. Catal. 2016, 333, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Wrigstedt, P.; Keskiväli, J.; Leskelä, M.; Repo, T. The Role of Salts and Brønsted Acids in Lewis Acid-Catalyzed Aqueous-Phase Glucose Dehydration to 5-Hydroxymethylfurfural. ChemCatChem 2015, 7, 501–507. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Navarro-Jaén, S.; Drault, F.; Ivanova, S. Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to Hmf and Lactic Acid: Pathways toward Bio-Based Plastics. Catalysts 2021, 11, 1395. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Su, Z.; De Oliveira Vigier, K.; Jérôme, F.; Poon, C.S.; Ok, Y.S. Organic Acid-Regulated Lewis Acidity for Selective Catalytic Hydroxymethylfurfural Production from Rice Waste: An Experimental-Computational Study. ACS Sustain. Chem. Eng. 2019, 7, 1437–1446. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Zhai, S.; Zhao, R.; Wang, J.; Cheng, X.; Shiran, H.S.; Larter, S.; Kibria, M.G.; Hu, J. Electron-Enriched Lewis Acid-Base Sites on Red Carbon Nitride for Simultaneous Hydrogen Production and Glucose Isomerization. Appl. Catal. B Environ. 2022, 316, 121647. [Google Scholar] [CrossRef]
- Lu, S.; Lyu, J.; Han, X.; Bai, P.; Guo, X. Effective Isomerization of Glucose to Fructose by Sn-MFI/MCM-41 Composites as Lewis Acid Catalysts. J. Taiwan Inst. Chem. Eng. 2020, 116, 272–278. [Google Scholar] [CrossRef]
- Das, B.; Mohanty, K. Sulfonic Acid-Functionalized Carbon Coated Red Mud as an Efficient Catalyst for the Direct Production of 5-HMF from D-Glucose under Microwave Irradiation. Appl. Catal. A Gen. 2021, 622, 118237. [Google Scholar] [CrossRef]
- Tempelman, C.; Jacobs, U.; Hut, T.; Pereira de Pina, E.; van Munster, M.; Cherkasov, N.; Degirmenci, V. Sn Exchanged Acidic Ion Exchange Resin for the Stable and Continuous Production of 5-HMF from Glucose at Low Temperature. Appl. Catal. A Gen. 2019, 588, 117267. [Google Scholar] [CrossRef]
- Huang, F.; Su, Y.; Tao, Y.; Sun, W.; Wang, W. Preparation of 5-Hydroxymethylfurfural from Glucose Catalyzed by Silica-Supported Phosphotungstic Acid Heterogeneous Catalyst. Fuel 2018, 226, 417–422. [Google Scholar] [CrossRef]
- Fachri, B.A.; Abdilla, R.M.; Bovenkamp, H.H.V.D.; Rasrendra, C.B.; Heeres, H.J. Experimental and Kinetic Modeling Studies on the Sulfuric Acid Catalyzed Conversion of d -Fructose to 5-Hydroxymethylfurfural and Levulinic Acid in Water. ACS Sustain. Chem. Eng. 2015, 3, 3024–3034. [Google Scholar] [CrossRef]
- Gomes, F.N.D.C.; Pereira, L.R.; Ribeiro, N.F.P.; Souza, M.M.V.M. Production of 5-Hydroxymethylfurfural (HMF) via Fructose Dehydration: Effect of Solvent and Salting-Out. Brazilian J. Chem. Eng. 2015, 32, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Abranches Dias Castro, G.; Lopes, N.; Fernandes, S.; da Silva, M. Copper Phosphotungstate-Catalyzed Microwave-Assisted Synthesis of 5-Hydroxymethylfurfural in a Biphasic System. Cellulose 2022, 29. [Google Scholar] [CrossRef]
- Castro, G.A.D.; Fernandes, S.A. Green Synthesis of 5-Hydroxymethylfurfural in a Biphasic System Assisted by Microwaves. Catal. Letters 2022. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Dumesic, J.A. Solvent Effects on Fructose Dehydration to 5-Hydroxymethylfurfural in Biphasic Systems Saturated with Inorganic Salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Görgényi, M.; Dewulf, J.; Van Langenhove, H.; Héberger, K. Aqueous Salting-out Effect of Inorganic Cations and Anions on Non-Electrolytes. Chemosphere 2006, 65, 802–810. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-Hydroxymethylfurfural Production from Biomass in Biphasic Solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J.; Leitner, W. An Overview of the Biphasic Dehydration of Sugars to 5-Hydroxymethylfurfural and Furfural: A Rational Selection of Solvents Using COSMO-RS and Selection Guides. Green Chem. 2020, 22, 2097–2128. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)Furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Vieira, J.L.; Paul, G.; Iga, G.D.; Cabral, N.M.; Bueno, J.M.C.; Bisio, C.; Gallo, J.M.R. Niobium Phosphates as Bifunctional Catalysts for the Conversion of Biomass-Derived Monosaccharides. Appl. Catal. A Gen. 2021, 617. [Google Scholar] [CrossRef]
- Ståhlberg, T.; Rodriguez-Rodriguez, S.; Fristrup, P.; Riisager, A. Metal-Free Dehydration of Glucose to 5-(Hydroxymethyl)Furfural in Ionic Liquids with Boric Acid as a Promoter. Chem.-A Eur. J. 2011, 17, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural from Glucose Using a Combination of Lewis and Brønsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Bounoukta, C.E.; Megías-Sayago, C.; Ammari, F.; Ivanova, S.; Monzon, A.; Centeno, M.A.; Odriozola, J.A. Dehydration of Glucose to 5-Hydroxymethlyfurfural on Bifunctional Carbon Catalysts. Appl. Catal. B Environ. 2021, 286, 119938. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Binder, J.B.; Cefali, A.V.; Blank, J.J.; Raines, R.T. Mechanistic Insights on the Conversion of Sugars into 5-Hydroxymethylfurfural. Energy Environ. Sci. 2010, 3, 765–771. [Google Scholar] [CrossRef]
- Scarim, C.B.; Lira de Farias, R.; Vieira de Godoy Netto, A.; Chin, C.M.; Leandro dos Santos, J.; Pavan, F.R. Recent Advances in Drug Discovery against Mycobacterium Tuberculosis: Metal-Based Complexes. Eur. J. Med. Chem. 2021, 214. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, F.; Wang, Y.T.; Len, C. Various Carbohydrate Precursors Dehydration to 5-HMF in an Acidic Biphasic System under Microwave Heating Using Betaine as a Co-Catalyst. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Hassan, E.B.; Steele, P. Rapid Conversion of Cellulose to 5-Hydroxymethylfurfural Using Single and Combined Metal Chloride Catalysts in Ionic Liquid. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2013, 41, 214–222. [Google Scholar] [CrossRef]
- Silvestri, C.; Silvestri, L.; Forcina, A.; Di Bona, G.; Falcone, D. Green Chemistry Contribution towards More Equitable Global Sustainability and Greater Circular Economy: A Systematic Literature Review. J. Clean. Prod. 2021, 294, 126137. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Gupta, D.; Saha, B. Dual Acidic Titania Carbocatalyst for Cascade Reaction of Sugar to Etherified Fuel Additives. Catal. Commun. 2018, 110, 46–50. [Google Scholar] [CrossRef]
- Noma, R.; Nakajima, K.; Kamata, K.; Kitano, M.; Hayashi, S.; Hara, M. Formation of 5-(Hydroxymethyl)Furfural by Stepwise Dehydration over TiO2 with Water-Tolerant Lewis Acid Sites. J. Phys. Chem. C 2015, 119, 17117–17125. [Google Scholar] [CrossRef]
- Parveen, F.; Upadhyayula, S. Efficient Conversion of Glucose to HMF Using Organocatalysts with Dual Acidic and Basic Functionalities-A Mechanistic and Experimental Study. Fuel Process. Technol. 2017, 162, 30–36. [Google Scholar] [CrossRef]
- Li, G.; Pidko, E.A.; Hensen, E.J.M.; Nakajima, K. A Density Functional Theory Study of the Mechanism of Direct Glucose Dehydration to 5-Hydroxymethylfurfural on Anatase Titania. ChemCatChem 2018, 10, 4084–4089. [Google Scholar] [CrossRef]
- Pidko, E.A.; Degirmenci, V.; Hensen, E.J.M. On the Mechanism of Lewis Acid Catalyzed Glucose Transformations in Ionic Liquids. ChemCatChem 2012, 4, 1263–1271. [Google Scholar] [CrossRef]
- Loerbroks, C.; Van Rijn, J.; Ruby, M.P.; Tong, Q.; Schüth, F.; Thiel, W. Reactivity of Metal Catalysts in Glucose-Fructose Conversion. Chem.-A Eur. J. 2014, 20, 12298–12309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román-Leshkov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of Glucose Isomerization Using a Solid Lewis Acid Catalyst in Water. Angew. Chemie-Int. Ed. 2010, 49, 8954–8957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutsche, C.; Iqbal, M. P-Tert-Buthylcalix[4]Arene. Org Synth 1990, 68, 234. [Google Scholar]
- Casnati, A.; Ca’, N.D.; Sansone, F.; Ugozzoli, F.; Ungaro, R. Enlarging the Size of Calix[4]Arene-Crowns-6 to Improve Cs+/K+selectivity: A Theoretical and Experimental Study. Tetrahedron 2004, 60, 7869–7876. [Google Scholar] [CrossRef]
- Shinkai, S.; Tsubaki, T.; Sone, T.; Manabe, O. A New Synthesis of P-Nitrocalix[6]Arene. Tetrahedron Lett. 1985, 26, 3343–3344. [Google Scholar] [CrossRef]






 | ||||
|---|---|---|---|---|
| Entry | CX4SO3H (% wt) | NbCl5 (% wt) | Temperature (°C) | Yield (%) |
| 1 | 5.0 | 0.0 | 150 | trace |
| 2 | 5.0 | 2.5 | 150 | 27 |
| 3 | 5.0 | 5.0 | 150 | 31 |
| 4 | 5.0 | 7.5 | 150 | 42 |
| 5 | 5.0 | 10.0 | 150 | 29 |
| 6 | 0.0 | 7.5 | 150 | 20 |
| 7 | 2.5 | 7.5 | 150 | 30 |
| 8 | 7.5 | 7.5 | 150 | 39 |
| 9 | 10.0 | 7.5 | 150 | 32 |
 | ||||
|---|---|---|---|---|
| Entry | CX4SO3H (% wt) | NbCl5 (% wt) | Temperature (°C) | Yield (%) |
| 1 | 5.0 | 7.5 | 130 | 21 |
| 2 | 5.0 | 7.5 | 140 | 30 |
| 3 | 5.0 | 7.5 | 150 | 42 |
| 4 a | 5.0 | 7.5 | 160 | 33 |
 | |||
|---|---|---|---|
| Entry | Phase | Yield (%) | |
| Aqueous | Organic | ||
| 1 | NaCl | MIBK | 50 |
| 2 | KCl | MIBK | 29 |
| 3 | CaCl2 | MIBK | 24 |
| 4 | MgCl2 | MIBK | 11 |
| 5 | NaCl | - | 3 |
| 6 | - | MIBK | - |
| Entry | Organic Phase/Reaction Phase (Ratio) | Catalyst | Experimental Conditions | Yield (%) | Ref |
|---|---|---|---|---|---|
| 1 | MIBK/Water a (3:1) | CX4SO3H/ NbCl5 | T = 150 °C cat = 1/1.5 wt% time = 17.5 min | 50 | This work |
| 2 | THF b/Water (4:1) | Nb2O5/HCl | T = 130 °C cat = ½ wt% time = 120 min | 47 | [58] |
| 3 | THF b/Water a (2:1) | CrCl3/HCl | T = 140 °C cat = 3/10 wt% time = 180 min | 59 | [59] |
| 4 | SCB c/Water a (2:1) | AlCl3/HCl | T = 170 °C cat = not reported * time = 40 min | 62 | [60] |
| 5 | MIBK/water a (6:1) | PTSA-Ca/AC | T = 180 °C cat = 1/1 time = 1440 min | 57 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, G.F.; Delgadillo, D.M.E.; Castro, G.A.D.; Cubides-Roman, D.C.; Fernandes, S.A.; Lacerda Júnior, V. Conversion of Glucose to 5-Hydroxymethylfurfural Using Consortium Catalyst in a Biphasic System and Mechanistic Insights. Catalysts 2023, 13, 574. https://doi.org/10.3390/catal13030574
David GF, Delgadillo DME, Castro GAD, Cubides-Roman DC, Fernandes SA, Lacerda Júnior V. Conversion of Glucose to 5-Hydroxymethylfurfural Using Consortium Catalyst in a Biphasic System and Mechanistic Insights. Catalysts. 2023; 13(3):574. https://doi.org/10.3390/catal13030574
Chicago/Turabian StyleDavid, Geraldo Ferreira, Daniela Margarita Echeverri Delgadillo, Gabriel Abranches Dias Castro, Diana Catalina Cubides-Roman, Sergio Antonio Fernandes, and Valdemar Lacerda Júnior. 2023. "Conversion of Glucose to 5-Hydroxymethylfurfural Using Consortium Catalyst in a Biphasic System and Mechanistic Insights" Catalysts 13, no. 3: 574. https://doi.org/10.3390/catal13030574
APA StyleDavid, G. F., Delgadillo, D. M. E., Castro, G. A. D., Cubides-Roman, D. C., Fernandes, S. A., & Lacerda Júnior, V. (2023). Conversion of Glucose to 5-Hydroxymethylfurfural Using Consortium Catalyst in a Biphasic System and Mechanistic Insights. Catalysts, 13(3), 574. https://doi.org/10.3390/catal13030574