Benzene Oxidation over Pt Loaded on Fly Ash Zeolite X
Abstract
:1. Introduction
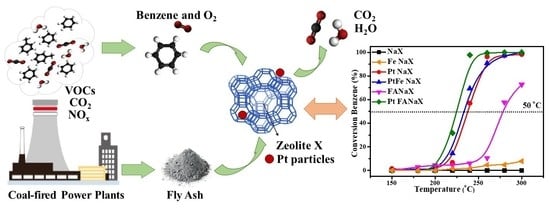
2. Results and Discussion
- (1)
- 3Fe2O3 + H2 → 2Fe3O4 + H2O
- (2)
- Fe3O4 + 4H2 → 3Fe + 4H2O
- (3)
- (1 − x)Fe3O4 + (1 − 4x)H2 → 3Fe(1 − x)O + (1 − 4x)H2O
- (4)
- Fe(1 − x)O + H2 → (1 − x)Fe + H2O
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babajide, O.; Musyoka, N.; Petrik, L.; Ameer, F. Novel zeolite Na-X synthesized from fly ash as a heterogeneous catalyst in biodiesel production. Catal. Today 2012, 190, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Ojha, K.; Pradhan, N.C.; Samanta, A.N. Zeolite from fly ash: Synthesis and characterization. Bull. Mater. Sci. 2004, 27, 555–564. [Google Scholar] [CrossRef]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The Recycling of Coal Fly Ash: A Review on Sustainable Developments and Economic Considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Ozturk, M.; Karaaslan, M.; Akgol, O.; Sevim, U.K. Mechanical and electromagnetic performance of cement based composites containing different replacement levels of ground granulated blast furnace slag, fly ash, silica fume and rice husk ash. Cem. Concr. Res. 2020, 136, 106177–106185. [Google Scholar] [CrossRef]
- Kalvachev, Y.; Todorova, T.; Popov, C. Recent progress in synthesis and application of nanosized and hierarchical Mordenite—A short review. Catalysts 2021, 11, 308. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Zeolite Science and Technology, 1st ed.; CRC Press: London, UK, 2003; pp. 1–1204. [Google Scholar]
- Querol, X.; Moreno, N.; Umana, J.C.; Alastuey, A.; Hernandez, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Zeolite synthesis from coal fly ash by hydrothermal reaction using various alkali sources. J. Chem. Technol. Biotechnol. 2002, 77, 280–286. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Beran, E.; Czímerová, A. Properties and potential applications of zeolitic materials produced from fly ash using simple method of synthesis. Powder Technol. 2006, 166, 47–54. [Google Scholar] [CrossRef]
- Kalvachev, Y.; Zgureva, D.; Boycheva, S.; Barbov, B.; Petrova, N. Synthesis of carbon dioxide adsorbents by zeolitization of fly ash. J. Therm. Anal. Calorim. 2016, 124, 101–106. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Miteva, S.; Marinov, I.; Behunová, D.M.; Trendafilova, I.; Popova, M.; Václaviková, M. Studies on the Potential of Nonmodified and Metal Oxide-Modified Coal Fly Ash Zeolites For Adsorption of Heavy Metals and Catalytic Degradation of Organics for Waste Water Recovery. Processes 2020, 8, 778. [Google Scholar] [CrossRef]
- Shokanov, A.; Vereshchak, M.; Manakova, I. Mössbauer and X-ray Studies of Phase Composition of Fly Ashes Formed after Combustion of Ekibastuz Coal (Kazakhstan). Metals 2020, 10, 929. [Google Scholar] [CrossRef]
- Spivey, J.J. Complete catalytic oxidation of volatile organics. Ind. Eng. Chem. Res. 1987, 26, 2165–2180. [Google Scholar] [CrossRef]
- Kong, F.; Li, G.; Wang, J.; Shi, Y.; Zhou, R. Promoting effect of acid sites in hierarchical porous Pt/ZSM-5 catalysts for low-temperature removal of VOCs. Appl. Surf. Sci. 2022, 606, 154888. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Alotaibi, M.T.; El-Bahy, S.M. CO oxidation and 4-nitrophenol reduction over ceria-promoted platinum nanoparticles impregnated with ZSM-5 zeolite. J. Rare Earths 2022, 40, 1247–1254. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Kong, F.; Zhou, R. Low-temperature VOCs oxidation performance of Pt/zeolites catalysts with hierarchical pore structure. J. Env. Environ. Sci. 2023, 124, 505–512. [Google Scholar] [CrossRef]
- Tsou, J.; Magnoux, P.; Guisnet, M.; Órfão, J.M.; Figueiredo, J.L. Catalytic oxidation of volatile organic compounds: Oxidation of methyl-isobutyl-ketone over Pt/zeolite catalysts. Appl. Catal. B Env. Environ. 2005, 57, 117–123. [Google Scholar] [CrossRef]
- Chen, C.; Chen, F.; Zhang, L.; Pan, S.; Bian, C.; Zheng, X.; Meng, X.; Xiao, F.S. Importance of platinum particle size for complete oxidation of toluene over Pt/ZSM-5 catalysts. Chem. Commun. 2015, 51, 5936–5938. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Gutiérrez-Ortiz, J.I.; Gutiérrez-Ortiz, M.A.; González-Velasco, J.R. Catalytic oxidation of aliphatic chlorinated volatile organic compounds over Pt/H-BETA zeolite catalyst under dry and humid conditions. Catal. Today 2005, 107–108, 200–207. [Google Scholar] [CrossRef]
- Kummer, J.T. Oxidation of CO and C2H4 by Base Metal Catalysts Prepared on Honeycomb Supports. In Advances in Chemistry; Evoy, M., Ed.; Series 143; American Chemical Society: Washington, DC, USA, 1975; pp. 178–192. [Google Scholar] [CrossRef]
- Walker, S.; Straguzzi, G.I.; Manogue, W.H.; Schuit, G.C.A. Carbon monoxide and propene oxidation by iron oxides for auto-emission control. J. Catal. 1988, 110, 298–309. [Google Scholar] [CrossRef]
- Li, P.; Miser, D.E.; Rabiei, S.; Yadav, R.T.; Hajaligol, M.R. The removal of carbon monoxide by iron oxide nanoparticles. Appl. Catal. B Environ. 2003, 43, 151–162. [Google Scholar] [CrossRef]
- Szegedi, A.; Hegedus, M.; Margitfalvi, J.L.; Kiricsi, I. Low temperature CO oxidation over iron-containing MCM-41 catalysts. Chem. Commun. 2005, 11, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Rangus, M.; Mazaj, M.; Dražić, G.; Popova, M.; Tušar, N.N. Active Iron Sites of Disordered Mesoporous Silica Catalyst FeKIL-2 in the Oxidation of Volatile Organic Compounds (VOC). Materials 2014, 7, 4243–4257. [Google Scholar] [CrossRef] [Green Version]
- Jianga, X.C.; Yu, A.B. Synthesis of Pd/α-Fe2O3 nanocomposites for catalytic CO oxidation. J. Mater. Process Technol. 2009, 209, 4558–4562. [Google Scholar] [CrossRef]
- Shao, J.; Zhai, Y.; Zhang, L.; Xiang, L.; Lin, F. Low-Temperature Catalytic Ozonation of Multitype VOCs over Zeolite-Supported Catalysts. Int. J. Environ. Res. Public Health 2022, 19, 14515. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Q.; Han, R.; Fu, K.; Su, Y.; Zheng, Y.; Wu, X.; Song, C.; Ji, N.; Lu, X.; et al. Confinement and synergy effect of bimetallic Pt-Mn nanoparticles encapsulated in ZSM-5 zeolite with superior performance for acetone catalytic oxidation. Appl. Catal. B Environ. 2022, 309, 121224. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Lian, H.; Jia, M.; Zhao, G.; Jiang, D.; Zhang, W. Low-temperature CO oxidation over supported Pt catalysts prepared by colloid-deposition method. Catal. Commun. 2008, 9, 1045–1049. [Google Scholar] [CrossRef]
- Li, J.; Xiao, G.; Guo, Z.; Lin, B.; Hu, Y.; Fu, M.; Ye, D. ZSM-5-supported V-Cu bimetallic oxide catalyst for remarkable catalytic oxidation of toluene in coal-fired flue gas. Chem. Eng. J. 2021, 419, 129675. [Google Scholar] [CrossRef]
- Jodaei, A.; Salari, D.; Niaei, A.; Khatamian, M.; Çaylak, N. Preparation of Ag–M (M: Fe, Co and Mn)–ZSM-5 bimetal catalysts with high performance for catalytic oxidation of ethyl acetate. Environ. Technol. 2011, 32, 395–406. [Google Scholar] [CrossRef]
- Shabani, J.M.; Ameh, A.E.; Oyekola, O.; Babajide, O.O.; Petric, L. Fusion-Assisted Hydrothermal Synthesis and Post-Synthesis Modification of Mesoporous Hydroxy Sodalite Zeolite Prepared from Waste Coal Fly Ash for Biodiesel Production. Catalysts 2022, 12, 1652. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.H. Conversion of coal fly ash into zeolite 4A and its applications in waste eater treatment and greenhouse gas reduction. In Proceedings of the IMECE2007, Seattle, WA, USA, 11–15 November 2007. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Vaclavıkova, M.; Kalvachev, Y.; Lazarova, H.; Popova, M. Studies on non-modified and copper-modified coal ash zeolites as heterogeneous catalysts forVOCs oxidation. J. Hazard. Mater. 2019, 361, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Boycheva, S.; Lazarova, H.; Zgureva, D.; Lázár, K.; Szegedi, Á. VOC oxidation and CO2 adsorption on dual adsorption/catalytic system based on fly ash zeolites. Catal. Today 2020, 357, 518–525. [Google Scholar] [CrossRef]
- Todorova, S.; Barbov, B.; Todorova, T.; Kolev, H.; Ivanova, I.; Shopska, M.; Kalvachev, Y. CO oxidation over Pt-modified fly ash zeolite X. React. Kinet. Mech. Cat. 2020, 129, 773–786. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. (Eds.) Collection of Simulated XRD Powder Patterns for Zeolites, Structure Commision of the International Zeolite Association, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Rayalu, S.; Udhoji, J.; Meshram, S.; Naidu, R.; Devotta, S. Estimation of crystallinity in fly ash-based zeolite-A using XRD and IR spectroscopy. Curr. Sci. 2005, 89, 2147–2151. Available online: https://www.jstor.org/stable/24111077 (accessed on 19 March 2023).
- Majchrzak-Kuceba, I. A simple thermogravimetric method for the evaluation of the degree of fly ash conversion into zeolite material. J. Porous Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Mos, Y.M.; Vermeulen, A.C.; Buisman, C.J.N.; Weijm, J. X-Ray Diffraction of Iron Containing Samples: The Importance of a Suitable Configuration. Geomicrobiol. J. 2018, 35, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ji, Y.; Qiao, Z.; Zhao, C.; He, J.; Zhang, H. Preparation, Characterization, and Application of Magnetic Fe-SBA-15 Mesoporous Silica Molecular Sieves. J. Anal. Methods Chem. 2010, 2010, 323509. [Google Scholar] [CrossRef] [Green Version]
- Ma, I.-K.; Rigolet, S.; Michelin, L.; Paillaud, J.-L.; Mintova, S.; Khoerunnisa, F.; Daou, T.; Ng, E. Facile and fast determination of Si/Al ratio of zeolites using FTIR spectroscope technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Ono, L.K.; Yuan, B.; Heinrich, H.; Cuenya, B.R. Formation and Thermal Stability of Platinum Oxides on Size-Selected Platinum Nanoparticles: Support Effects. J. Phys. Chem. C 2010, 114, 22119–22133. [Google Scholar] [CrossRef]
- Moulder, F.; Sticke, W.F.; Sobol, P.E.; Bombel, K.D. Handbook of X-ray Photoelectron Spectroscopy, 2nd ed.; Perkin-Elmer Corporation, Physical Electron Division: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Grunthaner, P.J.; Grunthaner, F.J.; Madhukar, A. Chemical bonding and charge redistribution: Valence band and core level correlations for the Ni/Si, Pd/Si, and Pt/Si systems. J. Vac. Sci. Technol. 1982, 20, 680–683. [Google Scholar] [CrossRef]
- Oku, M.; Hirokawa, K. X-ray photoelectron spectroscopy of Co3O4, Fe3O4, Mn3O4, and related compounds. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 475–481. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Salyn, Y.V.; Leonhardt, G.; Scheibe, R. A comparison of different spectrometers and charge corrections used in X-ray photoelectron spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 1977, 10, 121–124. [Google Scholar] [CrossRef]
- Allen, G.C.; Curtis, M.T.; Hooper, A.J.; Tucker, P.M. X-Ray photoelectron spectroscopy of iron–oxygen systems. J. Chem. Soc. Dalton Trans. 1974, 14, 1525–1530. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Hea, C.; Li, J.; Li, P.; Chenga, J.; Haoa, Z.; Xu, Z.-P. Comprehensive investigation of Pd/ZSM-5/MCM-48 composite catalysts with enhanced activity and stability for benzene oxidation. Appl. Catal. B Environ. 2010, 96, 466–475. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Mo, S.; Liu, H.; Chen, Y.; Yang, J. Nanodendritic Platinum Supported on γ-Alumina for Complete Benzene Oxidation. Part. Part. Syst. Charact. 2016, 33, 620–627. [Google Scholar] [CrossRef]
- Tabakova, T.; Ilieva, L.; Petrova, P.; Venezia, A.M.; Avdeev, G.; Zanella, R.; Karakirova, Y. Complete benzene oxidation over mono and bimetallic Au–Pd catalysts supported on Fe-modified ceria. Chem. Eng. J. 2015, 260, 133–141. [Google Scholar] [CrossRef]
- Ilieva, L.; Petrova, P.; Venezia, A.M.; Anghel, E.; State, R.; Avdeev, G.; Tabakova, T. Mechanochemically prepared Co3O4-CeO2 catalysts for complete benzene oxidation. Catalysts 2021, 11, 1316. [Google Scholar] [CrossRef]
- Tomatis, M.; Xu, H.; Wei, C.; Bishop, M.; He, J.; Wang, C.; Zhao, M.; Xiao, H.; Yu, H.; Behera, S.N.; et al. A Comparative Study of Mn/Co Binary Metal Catalysts Supported on Two Commercial Diatomaceous Earths for Oxidation of Benzene. Catalysts 2018, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Tabakova, T.; Ilieva, L.; Petrova, P.; Venezia, A.M.; Karakirova, Y.; Liotta, L.; Avdeev, G. Complete benzene oxidation over mono and bimetallic Pd-Au catalysts on Alumina supported Y-dopped ceria. Appl. Sci. 2020, 10, 1088. [Google Scholar] [CrossRef] [Green Version]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Monti, D.A.M.; Baiker, A. Temperature-programmed reduction. Parametric sensitivity and estimation of kinetic parameters. J. Catal. 1983, 83, 323–335. [Google Scholar] [CrossRef]








| Samples, Composition in wt. % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Na2O | Fe2O3 | CaO | K2O | MgO | MnO | P2O5 | TiO2 | Weight Loss on Calcination | |
| FA | 54.50 | 23.95 | 0.76 | 10.21 | 2.14 | 3.43 | 2.62 | 0.07 | 0.15 | 1.09 | 0.8 |
| NaX | 39.90 | 25.84 | 15.62 | - | - | - | - | - | - | - | 18.23 |
| FANaX | 36.64 | 23.07 | 14.38 | 4.50 | 1.71 | 0.58 | 2.15 | 0.06 | 0.02 | 0.77 | 16.02 |
| Sample | SBET a [m2/g] | Smi b [m2/g] | Vt c [cm3/g] | Vmi b [cm3/g] | Vsec d [cm3/g] |
|---|---|---|---|---|---|
| NaX | 559 | 496 | 0.41 | 0.27 | 0.14 |
| Pt NaX | 501 | 422 | 0.46 | 0.23 | 0.23 |
| Fe NaX | 136 | 18 | 0.24 | 0.01 | 0.23 |
| PtFe NaX | 127 | 23 | 0.19 | 0.01 | 0.18 |
| FANaX | 386 | 354 | 0.27 | 0.19 | 0.08 |
| Pt FANaX | 370 | 326 | 0.26 | 0.18 | 0.08 |
| Sample | C1s % | O1s % | Al2p % | Si2p % | Na1s % | Mg1s % | Ca2p % | K2p % | Pt4f % | Fe2p % | N1s % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NaX | 14.23 | 45.91 | 11.64 | 17.95 | 10.27 | - | - | - | - | - | - |
| Fe NaX | 6.30 | 53.78 | 11.58 | 19.51 | 6.59 | - | - | - | - | 1.50 | 6.59 |
| Pt NaX | 4.19 | 45.59 | 11.96 | 21.51 | 13.72 | - | - | - | 0.04 | - | - |
| PtFe NaX | 4.41 | 55.30 | 12.40 | 18.52 | 6.61 | - | - | - | 0.05 | 1.64 | 1.06 |
| FANaX | 5.18 | 50.47 | 7.88 | 19.16 | 11.17 | 2.05 | 1.19 | 0.44 | - | 2.46 | - |
| Pt FANaX | 4.95 | 48.74 | 8.83 | 18.53 | 10.75 | 2.38 | 2.84 | 0.41 | 0.02 | 2.56 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalvachev, Y.; Todorova, T.; Kolev, H.; Merker, D.; Popov, C. Benzene Oxidation over Pt Loaded on Fly Ash Zeolite X. Catalysts 2023, 13, 1128. https://doi.org/10.3390/catal13071128
Kalvachev Y, Todorova T, Kolev H, Merker D, Popov C. Benzene Oxidation over Pt Loaded on Fly Ash Zeolite X. Catalysts. 2023; 13(7):1128. https://doi.org/10.3390/catal13071128
Chicago/Turabian StyleKalvachev, Yuri, Totka Todorova, Hristo Kolev, Daniel Merker, and Cyril Popov. 2023. "Benzene Oxidation over Pt Loaded on Fly Ash Zeolite X" Catalysts 13, no. 7: 1128. https://doi.org/10.3390/catal13071128
APA StyleKalvachev, Y., Todorova, T., Kolev, H., Merker, D., & Popov, C. (2023). Benzene Oxidation over Pt Loaded on Fly Ash Zeolite X. Catalysts, 13(7), 1128. https://doi.org/10.3390/catal13071128